IT-2-P-6055 Visualizing and correcting dynamic specimen processes in TEM using a large-format Direct Detection Device
Multiple factors reduce the resolution and signal-to-noise ratio (SNR) of transmission electron microscopy (TEM) images, including the microscope instrumentation, dynamic specimen processes (e.g., drift, beam-induced motion, charging, radiation damage, etc.), and inefficient electron detectors.
With the goal of overcoming many of these obstacles, Direct Electron introduced the first large-format Direct Detection Device (DDD®) in 2008, as the culmination of academic and industrial partnerships. Development has culminated in the DE-20 (5k x 4k) in 2012 and the new DE-64 (8k x 8k) this year. These DDD cameras deliver dramatically improved performance compared to traditional electron detectors such as film or CCD cameras.
In addition to improved efficiency and resolution, the architecture of DDD cameras allows for continuous streaming of unbinned full-frame images at ~30 frames per second, with no dead time between consecutive frames. Many TEM methods require a static specimen image, such as low-dose electron cryo-microscopy of biological specimens. In these methods, dynamic specimen processes are detrimental, causing either non-isotropic resolution loss (i.e., specimen drift) or overall degradation of the SNR in each image (e.g., beam-induced motion, charging, radiation damage, etc.). We have developed methods and algorithms for exploiting the “movie mode” output from DDD cameras to correct for these dynamic processes and maximize the isotropic resolution and SNR of each image. Briefly, a “movie” is acquired of a specimen at 2-3× the normal total electron exposure. To correct specimen drift (which is consistent across the entire image), the frames from the movie are iteratively aligned, and to correct beam-induced specimen motion and charging (which are local effects that vary across the image), sub-regions for each frame are iteratively aligned. To correct radiation damage, low-pass filters are applied to each frame based on expected damage rate of the specimen.
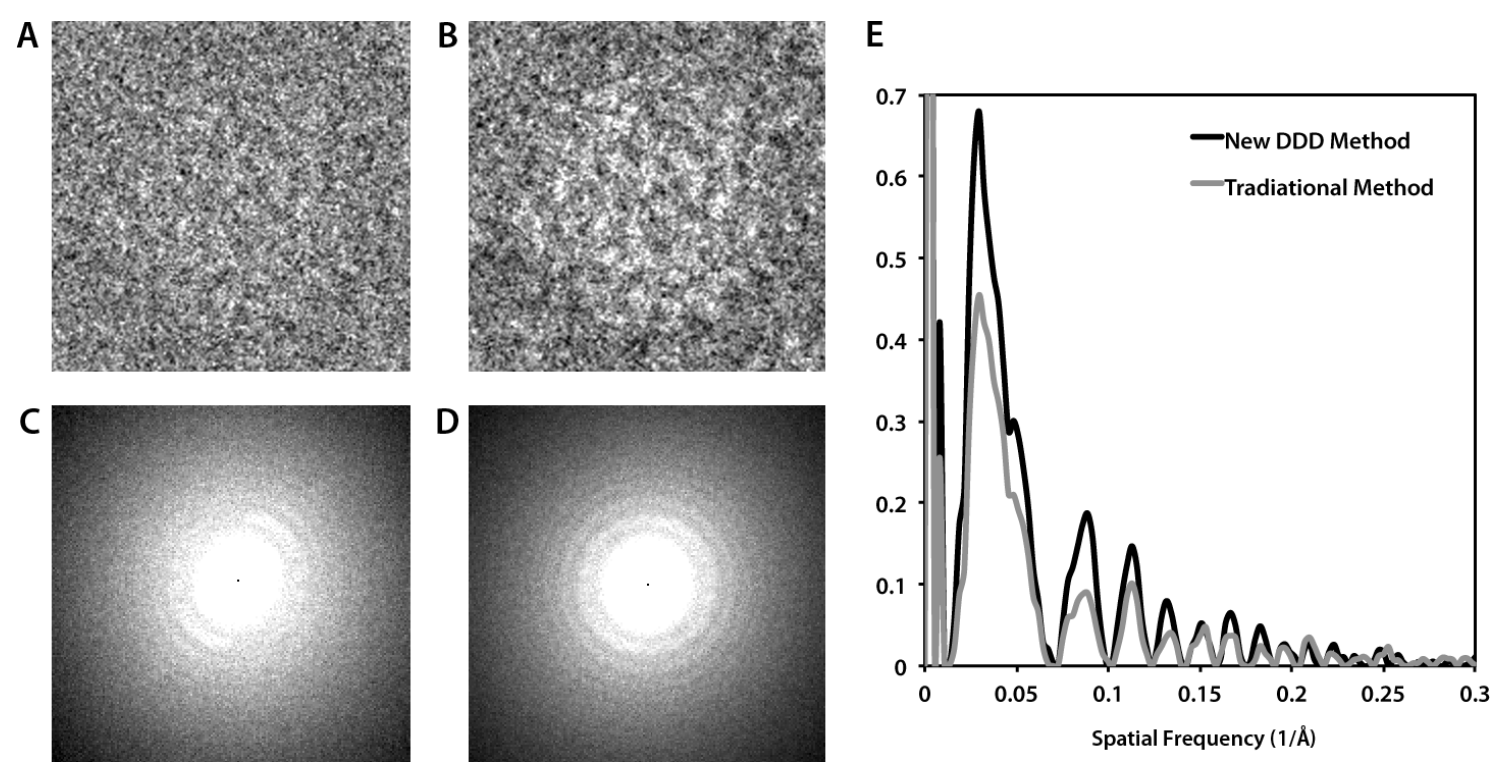
We have demonstrated the benefits of this method by using images of frozen-hydrated Brome mosaic virus (BMV). Images generated based on our method show improved isotropic high-frequency SNR along with significantly improved low-frequency contrast compared to conventional imaging (Fig. 1). We processed a data set of ~32,000 particles using both the conventional method and our new “damage compensation” method to generate de novo three-dimensional reconstructions of BMV. Our new method improved the resolution significantly from 4.4 Å to 3.8 Å resolution, thus demonstrating the power of damage compensation with a direct detection camera for high-resolution structural studies.
We sincerely thank the National Center for Macromolecular Imaging (Baylor College of Medicine, Houston, TX) for images and reconstructions of BMV. We also acknowledge funding from the National Institutes of Health (Grant #8R44GM103417-03).