IT-6-P-5740 Cultivation and Observation of HeLa Cells in the Microfluidic Environmental Electron Microscopy
Progress in the processing of wet tissues, without the need of fixation and other complex preparation, may facilitate the microscopic examination of tissues and cells. To solve the challenge that the moisture in the wet sample will be dried out by electron microscope’s vacuum system when observing the living cell, we attempt to develop an advanced MEMS wet-cell device with fluid-exchange to achieve the macromolecular dynamics observation accompanied with in-situ manipulating/monitoring under an electron microscope (EM), and then we report the observation of cells dynamics in solutions.
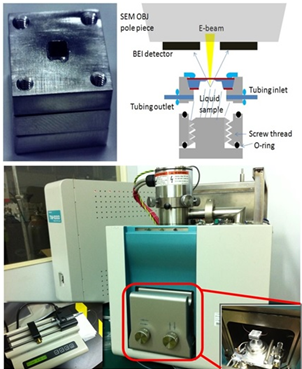
In this study, we design a special wet chamber (liquid SEM capsule) for environmental SEM by MEMS technology[1], consisted of one in-frame and one out-frame fitting to each other with controllable gap between for cell incubation and EM observation. In the current SEM application, the environmental wet chamber, composed of a disposable out-frame and a capsule, is inverted in SEM after sealing for the sample to facing up toward the incident electrons for getting stronger signals. Then, we connect the PEEK tubes to the capsule and use a syringe pump to provide liquid circulation (Figure1).
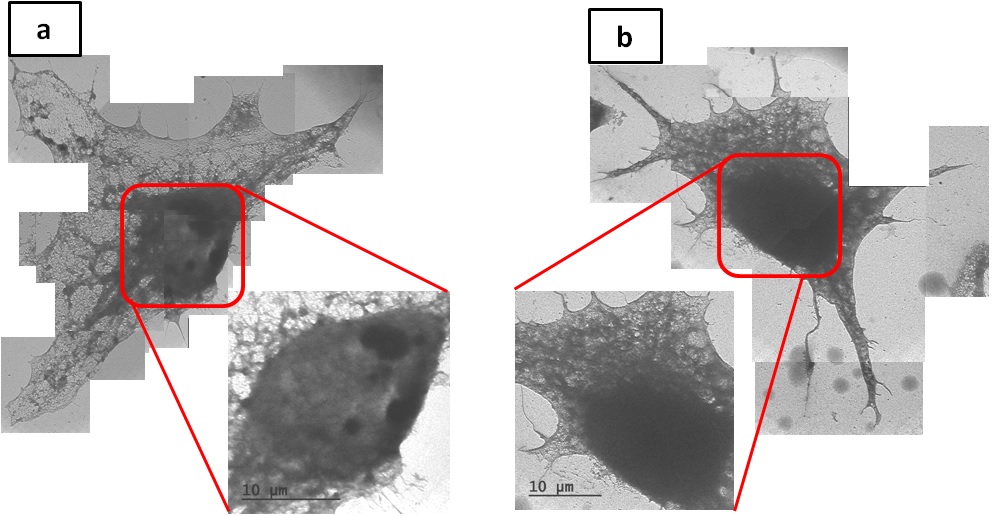
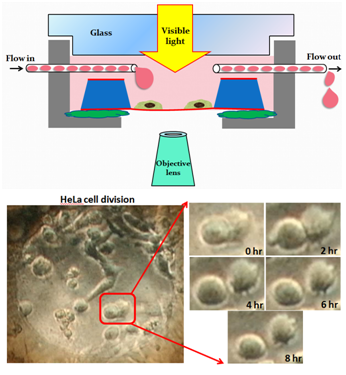
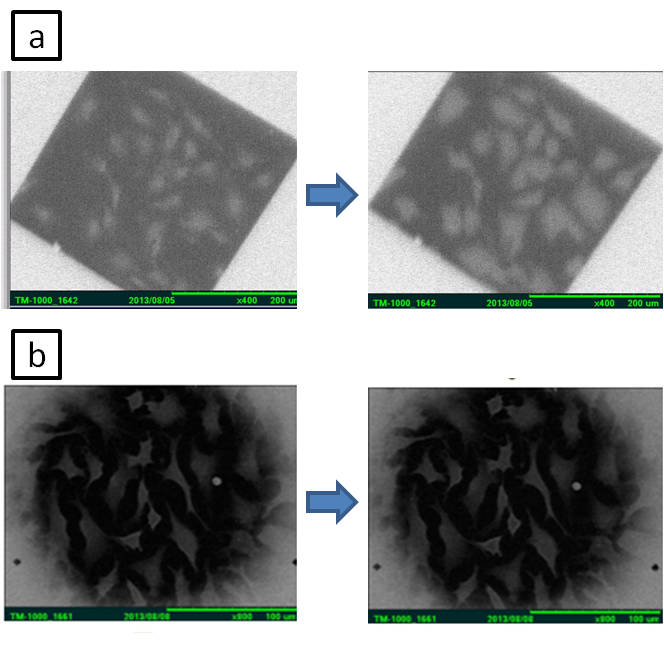
For living cell incubation inside wet cell, we immersed out-frame into culture dish to contain culture medium (DMEM with 10%FBS and 1%Penicillin/Streptomycin) , and then incubated HeLa cells for 8-12hr. To improve the image quality for the thick cell, we process cell permeabiliztion treatment (100%Methanol), immersing cell in the Milli-Q water in place of the cytoplasm. Comparing the image of different immersing time, we found that the two-hour immersion had a clearer view of cytoskeleton and nucleus (Figure2).Then we observed the living cell with our self-design component by fluid circulation way and recorded the cell division with slow flow rate(0.01ml/hr) under eight long hours OM observation (Figure3), which confirm the practicality of our design. Since the existence of liquid seriously influence the contrast, we replace the culture medium with glycerol, finding that the resolution is improved under long time SEM observation (Figure4). With the unique liquid circulating system incorporated with SEM, we can successfully incubate HeLa cells for a long period of time in the wet micro environment. The image resolution under a wet condition is characterized as 40-50 nm, suitable for observing interaction between virus and cells or subcell organelles.
1. ”Self-aligned wet-cell for hydrated microbiology observation in TEM.” T.W. Huang, S.Y. Liu, Y.J. Chuang, et al., Lab on a Chip.12:340-347(2012).
2. ”A Novel Method for Wet SEM,” Iris Barshack, Juri Kopolovic, Yehuda Chowers, Opher Gileadi, Anya Vainshtein, Ory Zik and Vered Behar, Ultrastructural Pathology, 28:29-31(2004).
This work was supported by National Science Council (NSC102-2321-B-007-007 and NSC 102-2120-M-007-006-CC1).