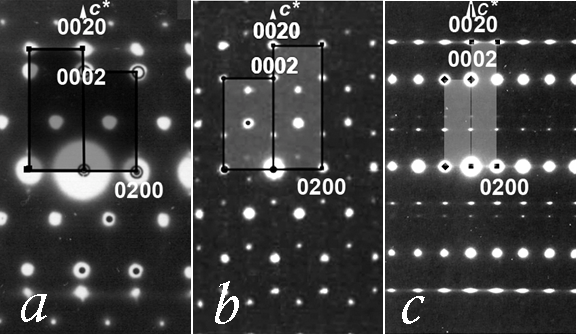
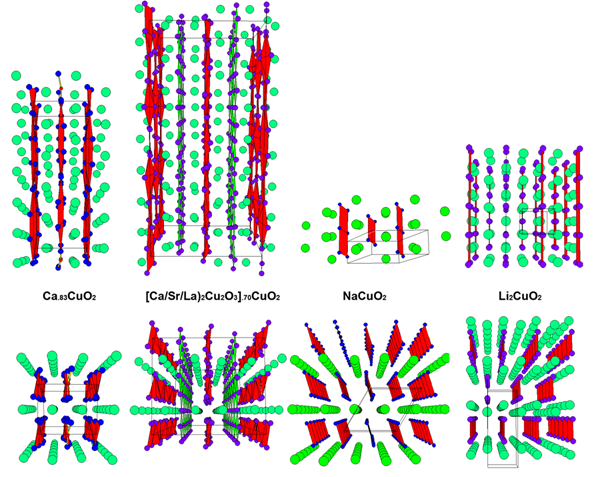
IT-9-P-3490 Composite crystal structures of MxCuO2 cuprates; (Mx = Li2, Ca.83, Sr.73, Ba.67, [(Sr/Ca)2Cu2O3]1/√2, Na)
The MxCuO2 cuprates belong to class of composite crystals consisting of two subsystems[1]: „CuO2-chains“ and „Mx-cations”. An electron microscopy and diffraction study of a number of rare earth cuprates, is presented (Fig.1.) in relation with the corresponding charge ordering that is induced by variable cation valency and nonstoichiometric composition. Level of cation defficiency and the accompanying self doping affects structure modulation, as well as the average Cu-valency; it can range from Cu+2.30 for Ca.83CuO2, to Cu+2.66 for Ba.67CuO2, and even up to Cu+3.0 for NaCuO2, or down to Cu+2.0 for Li2CuO2. In the case of Mx = Ca.83, Sr.73, Ba.67, [(Sr/Ca)2Cu2O3]1/√2, the two subsystems are mutually incommensurate and modulated along the “chain” direction, while for the end cases of: Mx = Na, Li2, the structural unit cells are commensurate (Fig. 2.)
The underlying lattices of these subsystems have common a and b parameters while the ratio cCh/cM of their c-parameters along the chain-direction varies with x. For a particular case of Mx=[(Ca/Sr)2Cu2O3]1/√2, the so-called “chain-ladder” (Sr/Ca)14Cu24O41 compound is well known for its optical and magnetic properties[2],[3]. In this case, the cation subsystem consists of an extended structure: (Sr/Ca)2Cu2O3 -“ladders”. The building unit of the ladders is a pair of cations plus a pair of zigzag edge-sharing CuO4-squares, Fig. 2, that are connected along “rungs”, so that the cLd period is defined by the CuO4-square diagonal. For the chains, the CuO4 building units share opposite edges and the cCh period is equal to the CuO4-square edge. In the case of “chain-ladder” composite structure, the cLd/cCh ratio is found to vary slightly with cation composition, but is always close to √2, so that the formula [(Ca/Sr)2Cu2O3]xCuO2 (x≈1/√2) correctly represents compound's composite structure.
With increasing Ca-substitution the cLd/cCh ratio varies from 1.44 for pure Sr14Cu24O41, to 1.416 for highly substituted Sr0.6Ca13.4Cu24O41. This is accompanied by charge (hole) redistribution between the CuO2-chains and the Cu2O3-ladders[3].
[1] Milat O., Van Tendeloo G., Amelinckx S., Mebhod M., Deltour R. (1992), Acta. Cryst., A48, 618-625.
[2] Vuletić T., Korin-Hamzić B., Ivek T., Tomić S., Gorshunov B., Dressel M., Akimitzu J. (2006), Phys. Rep. 428, 169-258.
[3] Ilakovac V., Gougoussis C., Calandra M., Brookes N. B., Bisogni V., Chiuzbaian S. G., Akimitsu J., Milat O., Tomic S., Hague C. F. (2012), Phy. Rev. B 85, 075108.
S. Tomić from Zagreb (Croatia), A. Migliori from Bologna (Italy), and G. Van Tendeloo from Antwerp (Belgium) are acknowledged for collacoration and support. Financial support has been received from Croatian Ministry of Sciences, Education and Sport.