IT-6-O-3407 Correlative Electron Microscopy and Photon Science Characterization of Working Catalysts
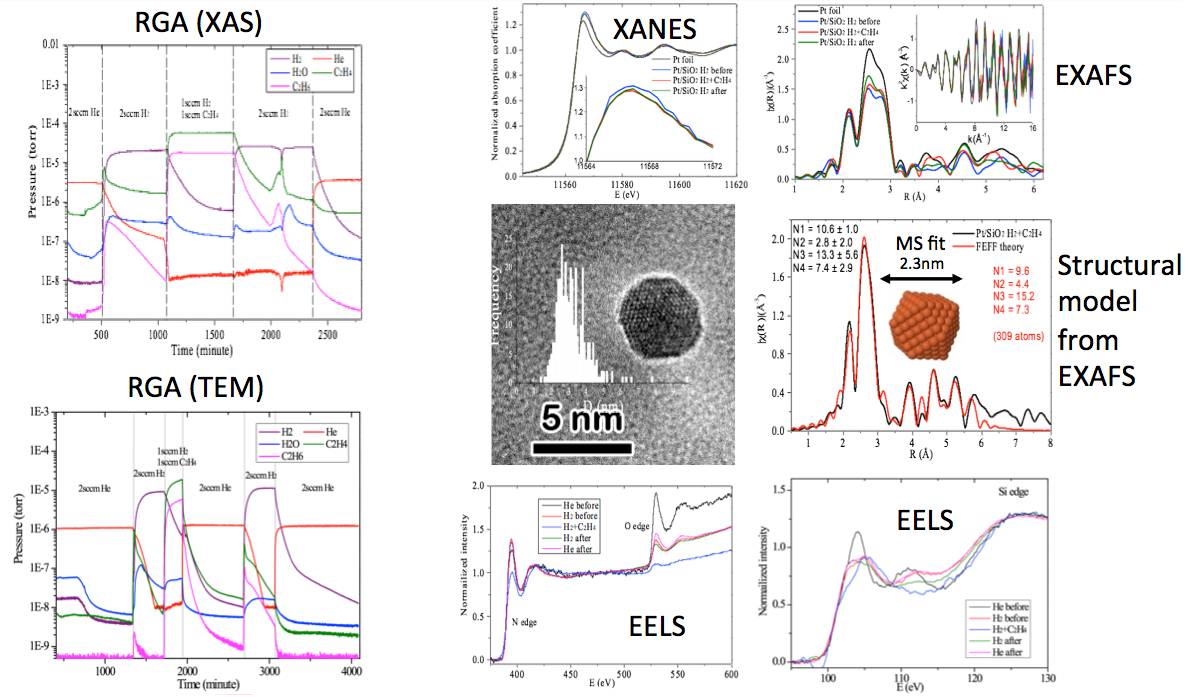
Characterization of catalytic reactions is often hindered by the fact that the behavior the system is mesoscopic, while the materials involved are nanoscale, with features that can span a broad range of temporal and spatial scales and which involve a broad range of competitive interactions. As a result, the description of a catalytic system requires interrogation with a variety of techniques – involving imaging, diffraction and spectroscopy – to describe the dynamic changes in structure that can occur during reactions. Commonly, this is done by simple use of standard techniques, and inference of how the results relate to the working condition of the system. It is, however, preferable that multiple probes are used to characterize physical and electronic structure of the catalyst during reaction, over multiple time and length scales. To date it has not been possible to directly link the observations across these techniques in such a way as to confirm that the data (imaging, diffraction, spectroscopy) is obtained from the system in the exact same “working” state. Here we report an experimental approach that allows: (1) characterization – via x-ray absorption spectroscopy, extended x-ray absorption fine structure, x-ray fluorescence, Raman spectroscopy, transmission electron microscopy, scanning transmission electron microscopy, electron energy loss spectroscopy and energy dispersive electron microscopy – from the same sample, (2) characterization at atmospheric pressures in reactive environments, and (3) simultaneous, real-time and on-line analysis of the reaction products – i.e. “operando” experimentation.
We take advantage of recent developments in sample holders for transmission electron microscopy that allow catalysts to be confined between two, thin nitride membrane supports that are separated by a narrow gap, and that allow continuous flow of liquid or gas through the system. We exploit the simplicity of this system in such a way as to allow utilization in both synchrotron x-ray beamlines and transmission electron microscopes. We have chosen a simple, model catalyst reaction for the demonstration phase of this work, the catalyzed conversion of ethylene to ethane, though the use of Pd/SiO2 and Pt/SiO¬2 heterogenous catalysts. Extension to high-temperature experimentation will be reported, thereby demonstrating the extension of this approach to the full class of catalytic systems.
Research carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. Y.L and A.F.F acknowledge additional support through the Synchrotron Catalysis Consortium, U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-FG02-03ER15476.