IT-7-P-3273 Atomic Level In-situ Characterization of NiO-TiO2 Photocatalysts under Light Irradiation in Water Vapor
Photocatalysts have potential applications for solar fuel generation either through water splitting. It is now recognized that atomic level in situ observations are critical for understanding the structure-reactivity in photocatalysts in the presence of reactant and product species and during in-situ light illumination. NiO loaded semiconductor photocatalysts with Ni first reduced and then partially re-oxidized at the surface has been reported to have good photocatalytic properties by forming a metallic Ni ohmic contact between NiO and the semiconductors [1]. TiO2 is a promising photocatalyst which has attracted intense research interest for decades since photo-decomposition of water by TiO2 was discovered. The TiO2 photocatalysts are either anatase or rutile which has been well known. Herein we use anatase as a model material to develop in situ photocatalytic experimental methodology and explore structure changes of NiO/semiconductor photocatalysts. In-situ heat treatment in H2 or O2 is applied to prepare initially Ni/TiO2, NiO/TiO2 or NiO-Ni-TiO2 materials in an environmental transmission electron microscope (ETEM). Then, without exposure to air, analysis can be performed in the same modified ETEM under in situ conditions in the presence of light and reactants to explore oxidation/reduction or interface changes under photocatalytic reactions.
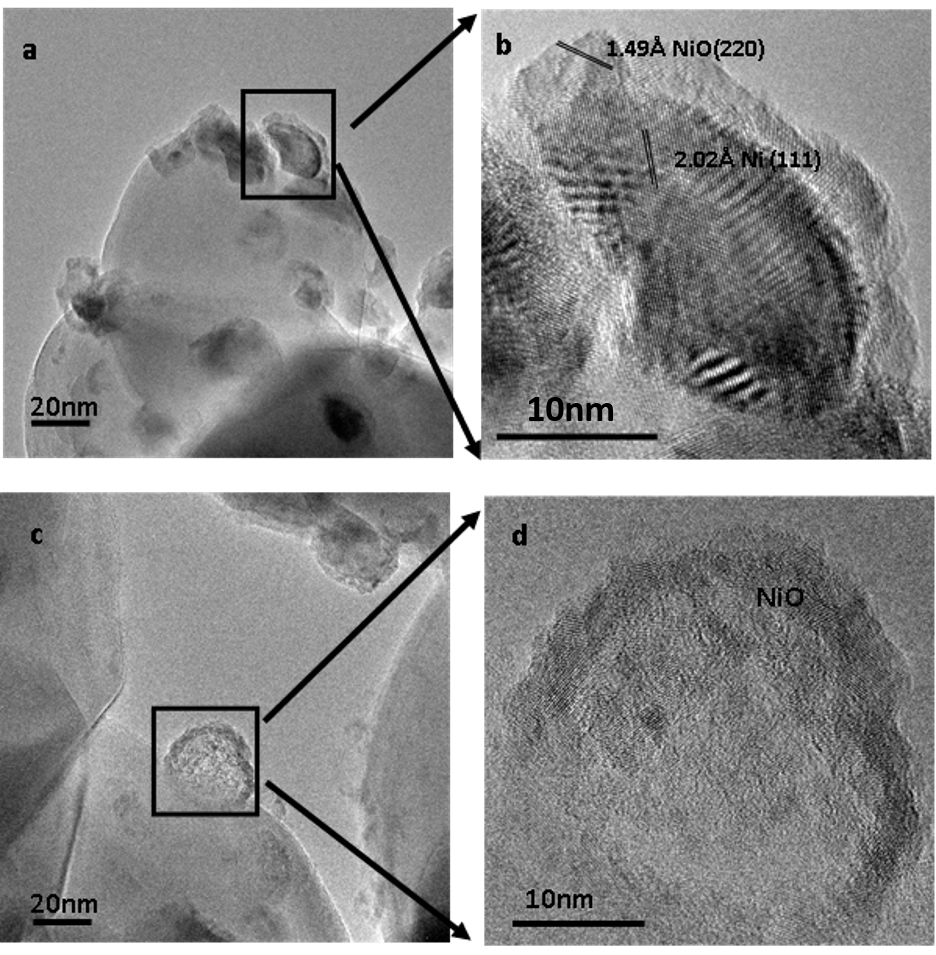
NiO-Ni-TiO2 was prepared using Ni(NiO3)2 as the precursor following impregnation, calcination, reduction and partial re-oxidation. Ex-situ experiments were performed to achieve preliminary observations under exposure of xenon lamp with mirror reflecting light in the range 360nm to 460nm light. TEM images for ex-situ experiments were recorded with a FEI aberration corrected Titan TEM. Figure 1A&1B show initial Ni-NiO core-shell structures on anatase particles. The inside rounded darker particles are Ni metals with outside shells of polycrystal NiO. After 6 hrs exposure to light in liquid water the oxide shells become porous and the Ni metal is absent leaving a void (Figure 1C&1D). Ni may either be oxidized to NiO or dissolved into the solution during photocatalytic reactions.
In-situ heat treatments using a hot stage sample holder with H2 or O2 allows Ni or NiO to be prepared as the starting material for in situ photocatalytic experiments. A FEI Tecnai F20 ETEM was modified to allow samples to be illuminated with light from a broadband laser driven light source (EQ-99, Energetiq Inc.) with the intensity up to 10 suns [2]. Changes taking place in these Ni metal and NiO structures under in situ light exposure in presence of water vapor will also be discussed.
References:
[1]. Domen, K.; Kudo, A.; Onishi, T.; J. Catalysis, 1986. 102,92-98
[2]. Miller, B.K.; Crozier, P.A. Microscopy and Microanalysis 2013, 19, 461-469
The support from US Department of Energy (DE-SC0004954) and the use of ETEM at John M. Cowley Center for HR Microscopy at Arizona State University is gratefully acknowledged.