IT-7-O-3169 Temperature-induced sphere-to-tetrapod transformation of CdSe nanocrystals investigated by in-situ transmission electron microscopy
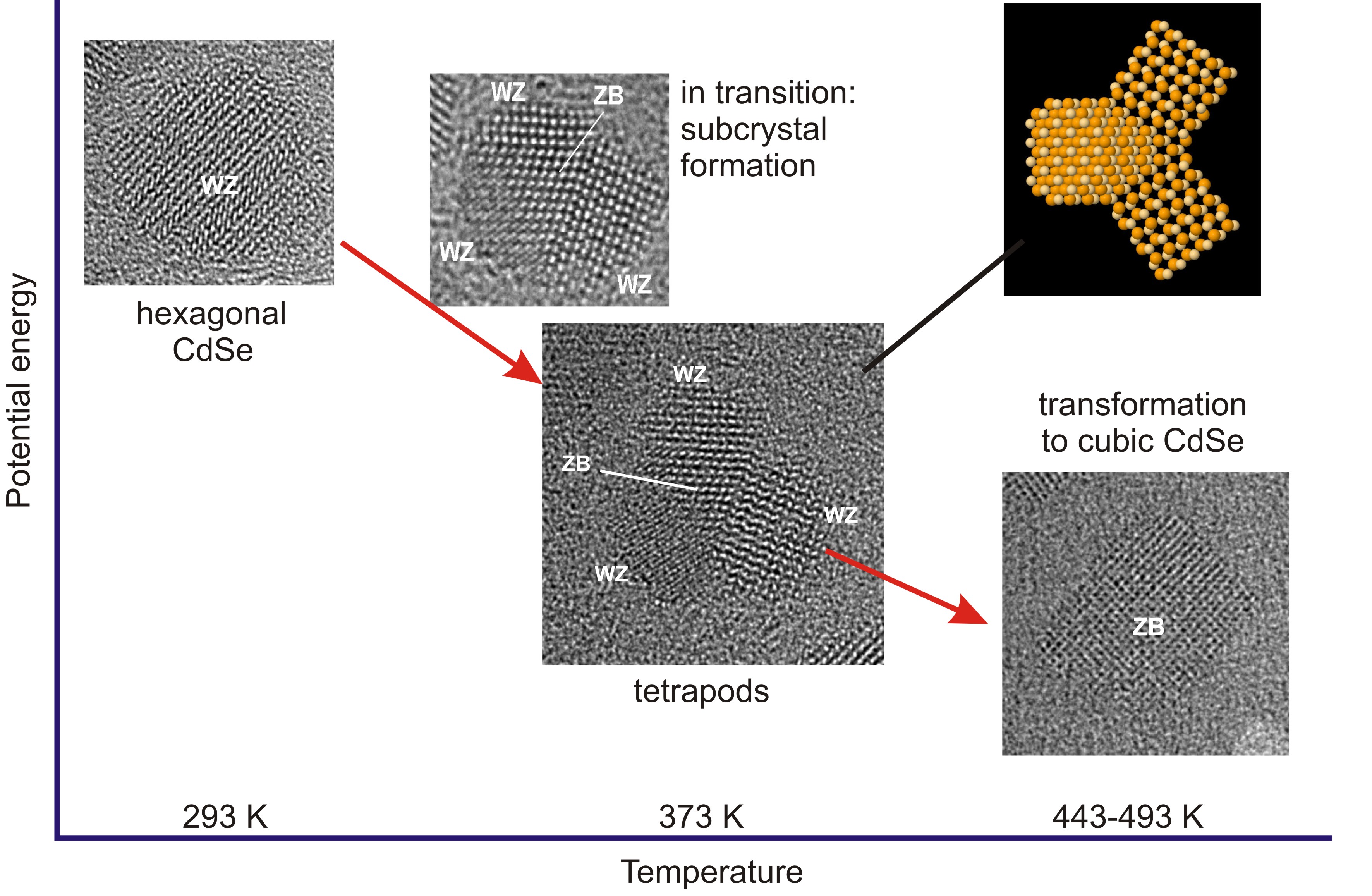
Colloidal CdSe nanocrystals (NCs) can be synthesized in a wide variety of (heterogeneous) nanostructures including sphere, rod, tetrapod, and octapod morphologies. Using a low-drift TEM heating holder [1] employing MEMS microheaters with 15 nm thick SiN windows, the thermal evolution of spherical CdSe NCs was followed in real time and with atomic resolution. With increasing temperature, the NCs were found to transform from spheres to multipods to rectangular single crystals. The thermal evolution is shown schematically in Figure 1.
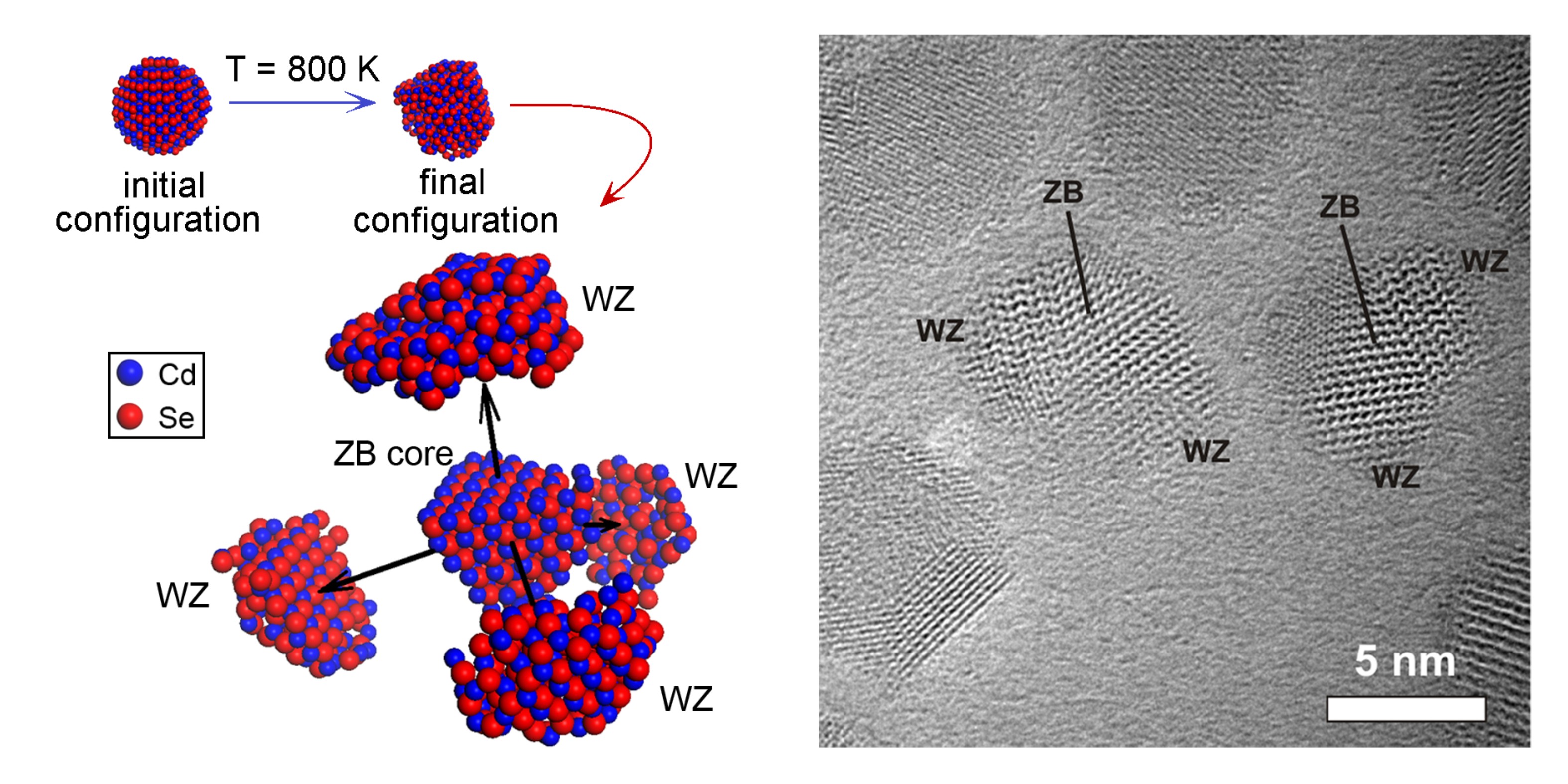
The as-synthesized CdSe NCs consist of multiple subcrystals, but are spherical in shape. Upon heating to a temperature of 80 °C, most NCs transform into bipods, tripods, or tetrapods, whereby the core exhibits the zinc blende (ZB) crystal structure while the pods have the wurtzite (WZ) crystal structure [2]. These multipods are remarkably stable, up to a temperature of 300 °C, as long as they remain isolated. Multipods that are close together, though, fuse into rectangular single crystals having the ZB structure at temperatures of 170-200 °C. This is an unexpected result, as the stable bulk phase of CdSe is WZ. The ZB NCs undergo multiple crystal fusion events by oriented attachment, which was recorded in real time and with atomic resolution. The fusion is followed by coalescence into larger agglomerates which eventually transform to the WZ crystal structure. The last step in the thermal evolution is sublimation which takes place at temperatures of 360‒400 °C.
Force-field molecular dynamics (FF-MD) simulations [2] (Figure 2), and density functional theory (DFT) calculations were performed in order to investigate the driving forces inducing these most remarkable transformation phenomena. It is concluded that off-stoichiometry can slightly favor the bulk ZB phase with respect to the bulk WZ phase, but that temperature-dependent interface-related energies are most likely the cause of the rich thermal behavior. Furthermore, it becomes clear that the ZB-WZ transformations are mediated by vacancies on the {111}Cd or {0001}Cd atomic planes.
[1] M.A. van Huis, N.P. Young, G. Pandraud, J.F. Creemer, D. Vanmaekelbergh, A.I. Kirkland, H.W. Zandbergen, ‘Atomic imaging of phase transitions and morphology transformations in nanocrystals’, Adv. Mater. 21 (2009) 4992-4995.
[2] Z. Fan, A.O. Yalcin, F.D. Tichelaar, H.W. Zandbergen, E. Talgorn, A.J. Houtepen, T.J.H. Vlugt, M.A. van Huis, ‘From sphere to multipod: thermally induced transitions of CdSe nanocrystals studied by molecular dynamics simulations’, J. Am. Chem. Soc. 135 (2013) 5869-5876.