IT-5-P-2963 TEM-EELS/SXES studies on electronic structures of p-type CaB6
Metal hexaboride MB6 is based on a network of B6-clusters located on each corner of cubic unit cell. M atom occupies at the body center position of the unit cell. When an M atom can supply two electrons to B6-network, the valence bands (VB) of this material is fully occupied and becomes a semiconductor. Those semiconductor materials have been investigated as a candidate for a high-temperature thermoelectric-power material. Seebeck coefficients of MB6 (M=Ba, Sr, Ca) synthesized by solid-state reaction method were reported to be negative, indicating those are n-type materials [1]. Recently, the p-type character for CaB6 synthesized from the mixture of CaCl2 and NaBH4 in eutectic LiCl-KCl molten salt was reported [2]. Thus, the electronic structure of this new material has been studied by using electron energy-loss spectroscopy (EELS) [3] and soft-X-ray emission spectroscopy (SXES) [4], which are methods for probing over and below the Fermi energy level, respectively.
EDS analysis of the p-type CaB6 showed an inclusion of a few % of Na. Electron diffraction patterns showed a good crystalline order. As one Na atom can transfer one electron to B6-network, Na-doping can be a hole-doping based on the rigid band structure scheme when doped Na atoms occupy Ca site. Valence electron excitation (from VB to conduction bands: CB) EELS spectra showed smaller bandgap energy of 1.5 eV than 2.5 eV of n-type CaB6 synthesized by solid-state reaction method. B K-shell excitation EELS spectra of p- and n-type materials showed almost the same onset energy, which energy position corresponds to the bottom of CB.
Figure 1 shows B K-emission SXES spectra of p-type and n-type CaB6. Those spectra show different intensity distribution especially at the top region of VB, which correspond to the right hand side end of the intensity distribution. The intensity distribution of p-type material apparently extends into the bandgap region of n-type CaB6. Since the bottom of CBs of the two materials were the same, this higher energy position of the top of VB of p-type should be the origin of the smaller bandgap energy of p-type material. This is consistent with the result of valence excitation EELS experiment stated above. This indicates that the doping of Na atoms into the Ca site of CaB6 causes not only the creation of holes in VB but also a change the energy state at the top region of VB, not a simple rigid band structure scheme.
[1] M.Takeda et al., J. Solid State Chemistry, 179, 2823-2826 (2006).
[2] K.Inayoshi and M. Takeda, IUMRS-ICEM (2012).
[3] Y.Sato et al., Ultramicroscopy 111, 1381-1387 (2011)
[4] M.Terauchi et al, Journal of Electron Microscopy 61, 1-8 (2012).
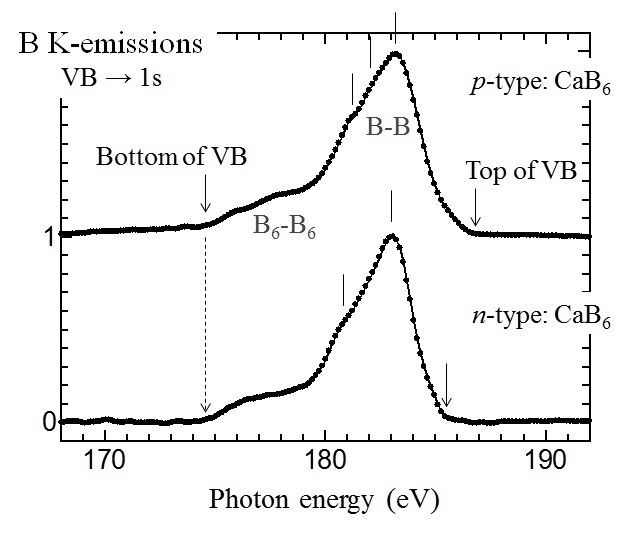
Fig. 1: B K-emission SXES spectra of p-type and n-type CaB6. |