IT-7-O-2952 In situ TEM observation of electrochemical deposition process
Recently, in situ transmission electron microscope (TEM) observations of lithiation and delithiation processes in lithium ion battery have been achieved in order to improve the performance. However, the electrolyte is liquid in conventional lithium ion battery. In order to observe the lithiation and delithiation processes in situ, it is necessary to develop an electrochemical cell, which keeps the liquid electrolyte in vacuum.
In this study, we developed an electrochemical cell with three terminals (working, reference, and counter electrodes) and demonstrated the process of electrochemical copper deposition on gold surfaces in-situ. Figure 1 shows a photograph of our home-made liquid cell. It is composed of two quartz glass pieces which are glued by a heat-curing epoxy with each other. The observation window is covered with a 50 nm silicon nitride film to keep the liquid inside. The advantage of our cell is that it is available to arrange suitable materials as cathode or anode. In this observation, the working, reference and counter electrodes were gold (Au), gold (Au) and copper (Cu), respectively. The electrolyte contained 0.2M CuSO4 and 0.05 M H2SO4. Cyclic voltammetry (CV) was obtained by using a VersaSTAT4 with a scan rate of 25 mV/ s.
Figure 2 shows a series of TEM images taken during CV measurement and the corresponding CV curve. Darker background corresponds to the deposited Au thin film of about 30 nm in thickness. We observed that Cu clusters were nucleated on the Au film when the bias voltage was negative, while they were desorbed when the voltage was positive. And also, during measuring CV repeatedly, we observed that Cu clusters were nucleated at the same position, corresponding to dots of slightly darker contrast as shown in the TEM image of (a). We consider that these dots correspond to the position where gold atoms were alloyed with copper atoms.
In conclusion, we have developed a new electrochemical cell for in situ TEM observation. Using the liquid cell, we have demonstrated electrochemical Cu deposition process on thin Au film simultaneously with measuring cyclic voltammetry.
This research was supported by Japan Science and Technology Agency (JST).
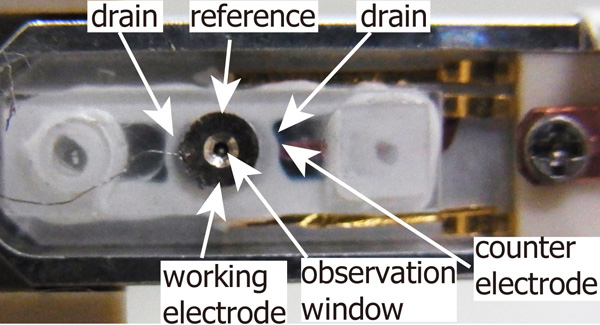
Fig. 1: A photograph of our developed electrochemical cell. |
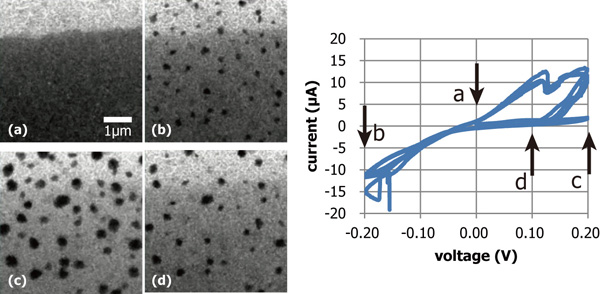
Fig. 2: (a)-(d) A series of TEM images taken during measuring cyclic voltammetry. Graph of cyclic voltammetry curves. |