IT-7-O-2947 In Situ Analytical Electron Microscopy: Imaging and Analysis of Steel in Liquid Water
In situ transmission electron microscopy has become an increasingly important and dynamic research area in materials science with the advent of unique microscope platforms and a range of specialized in situ specimen holders. In metals research, the ability to image and perform x-ray energy dispersive spectroscopy (XEDS) analyses of metals in liquids are particularly important for detailed study of the metal-environment interactions with specific microstructural features. We have recently demonstrated that both STEM imaging and XEDS data can be successfully obtained from nanoparticles in liquid in an aberration-corrected FEI Titan G2 S/TEM with Super EDX [1] [2]. Furthermore, a special hybrid specimen preparation technique involving electropolishing and FIB extraction has been developed to enable metal specimens to be studied in the liquid cell TEM specimen holder [3]. We have applied these techniques to examine austenitic stainless steel in distilled H2O.
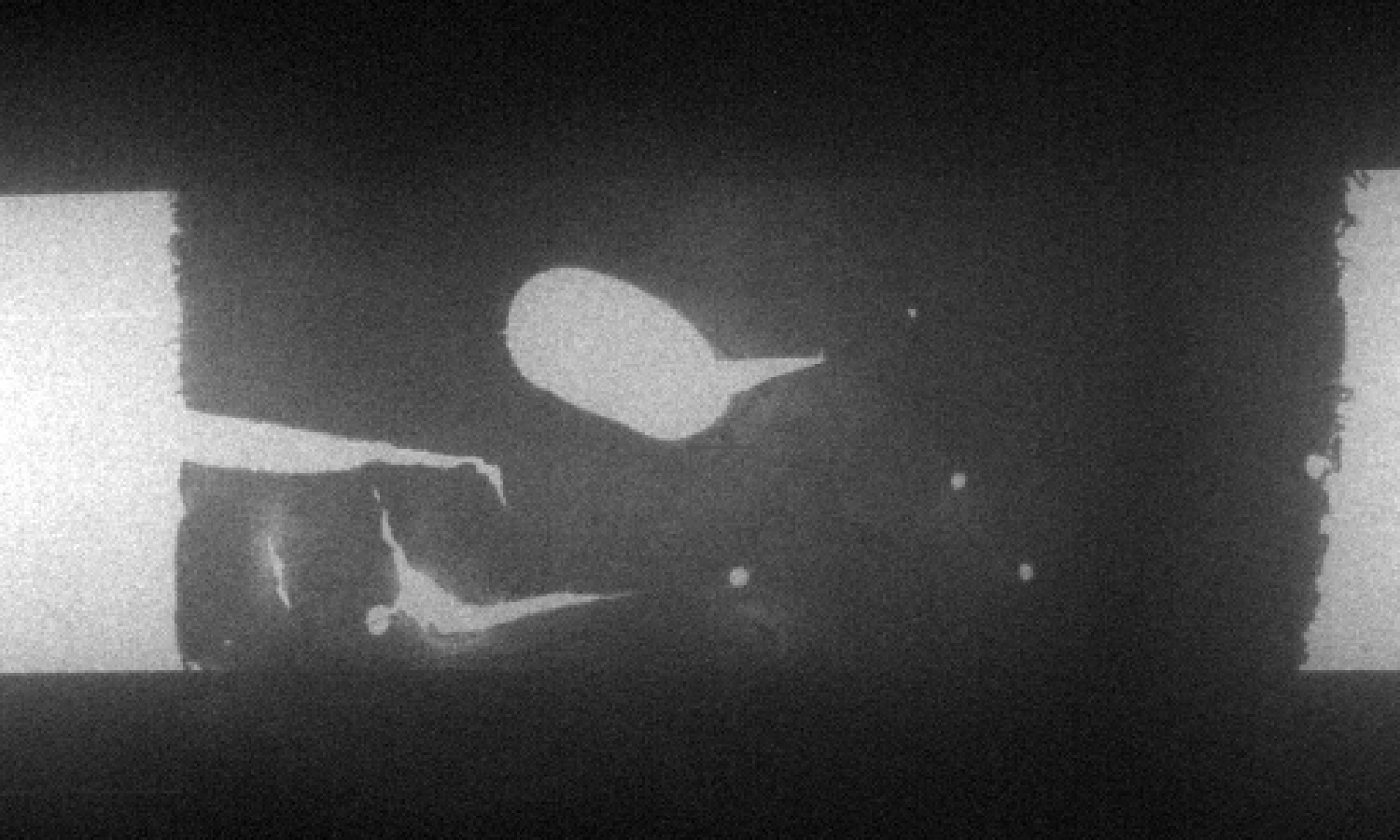
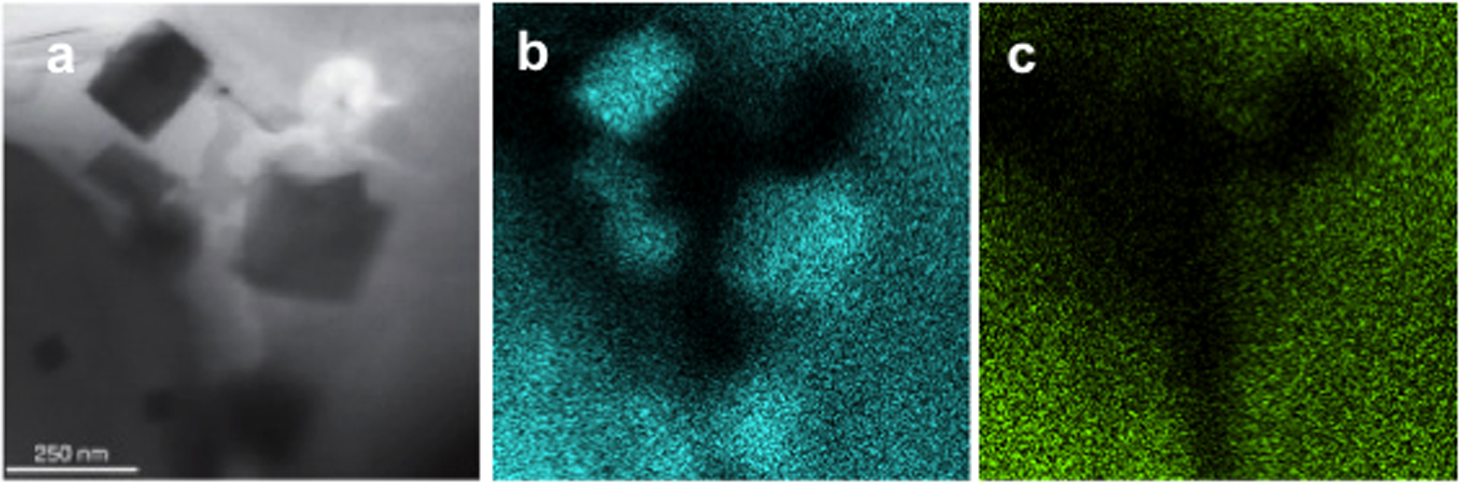
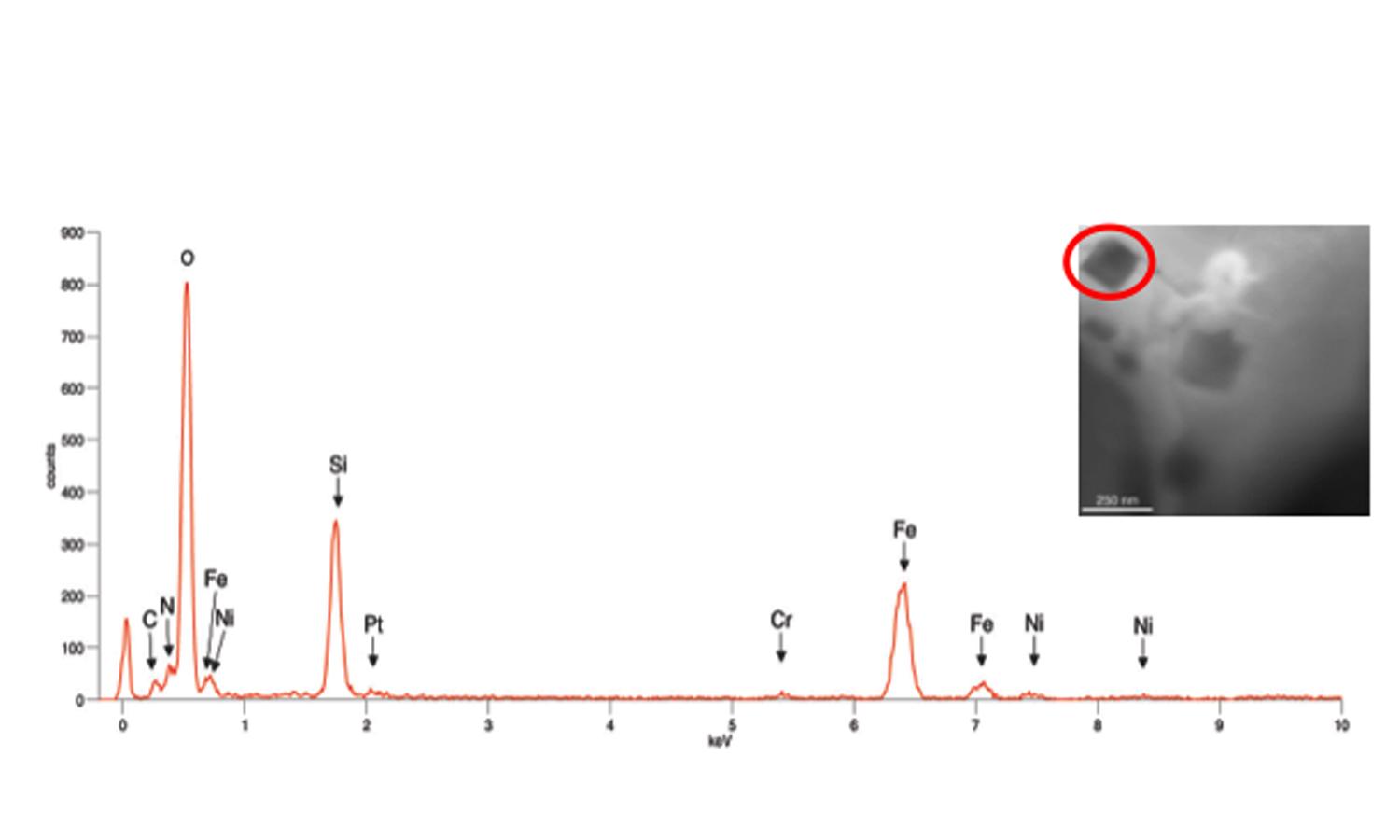
Conventional Type 304 austenitic stainless steel was prepared for examination in a Protochips Poseidon P200 liquid cell specimen holder with a 500 nm gap between the amorphous SiN windows. This specimen holder had been modified to optimize it for XEDS microanalysis [1]. TEM/STEM examination was performed using an FEI Tecnai T20 analytical electron microscope operated at 200 kV, equipped with an Oxford Instruments Xmax80TLE windowless Silicon Drift Detector (SDD) for XEDS spectrum imaging and analysis. Fig. 1 shows the Type 304 steel specimen imaged in distilled H2O. Fig. 2a shows several crystalline particles that were observed after 24 hours in H2O. Spectrum images (Fig. 2b,c) obtained from this area revealed that these particles were enriched in Fe and depleted in Cr, and were consistent with the formation of an Fe-rich oxide. An XED spectrum (Fig. 3) obtained from the coarse angular oxide demonstrated that the particle was Fe-rich but also contained low levels of Ni. These Fe-rich oxides can form because the thin Cr2O3 film formed on the Type 304 foil surfaces depletes the matrix of Cr. Thus, any defects in the passive film will enable the local Cr-depleted matrix to oxidise, thereby forming Fe-rich oxides. Further studies on the development of surface oxides and coarse oxide particles can aid in the study of passive film development in steels.
References
1. Zaluzec, N.J. et al., X-ray Energy-Dispersive Spectrometry During In Situ Liquid Cell Studies Using an Analytical Electron Microscope. Microsc. Microanal. 20, in press doi:10.1017/ S1431927614000154 (2014).
2. Lewis, E.A. et al., Wet Chemistry goes Nano. Submitted to Nanotechnology Letters.
3. Zhong, X.L. et al., Novel Hybrid Sample Preparation Method for In Situ Liquid Cell TEM Analysis. Submitted to Microsc and Microanal 2014.
The authors thank the BP 2013 DRL Innovation Fund, US DoE, Office of Basic Energy Sciences, and Contract No. DE-AC02-06CH11357 at the EM Center of Argonne National Laboratory.