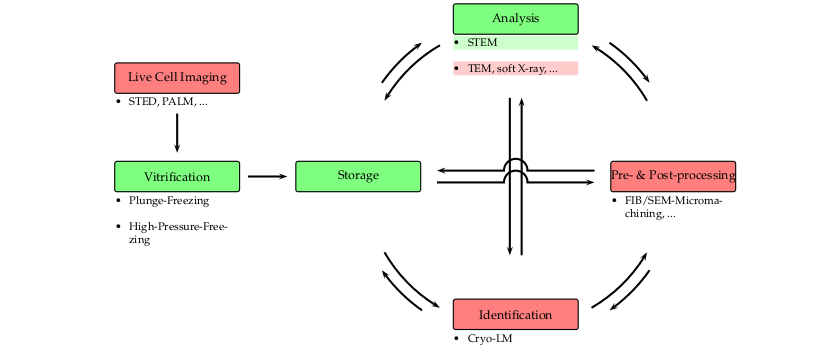
IT-7-O-2861 A cryo high-vacuum shuttle for correlative cryogenic investigations
The preservation of the native state is the key element in sample preparation. In the case of hydrated objects, embedding in vitreous (amorphous) ice and subsequent examination under cryogenic (cryo) conditions are the means of choice [1,2]. Over the last years, cryogenic techniques such as cryo-electron microscopy (cryo-EM) or soft X-ray cryo-microscopy have become increasingly popular, as they provide a direct, unaltered view on the specimen [3,4].
However, to provide a snapshot of the pristine architecture of the specimen, cryo techniques require constant cooling below the recrystallization temperature of 138°K [1] and avoidance of any contamination. This has been proven to be particularly challenging in the case of correlative cryo investigations [4,5], since these methods include several transfer steps due to their extensive post-processing [6] and complex workflow [7]. In the past, several transfer concepts were introduced and they are now commercially available. However, these systems are limited either by not offering a high-vacuum environment or constraining the applications to a restricted workflow.
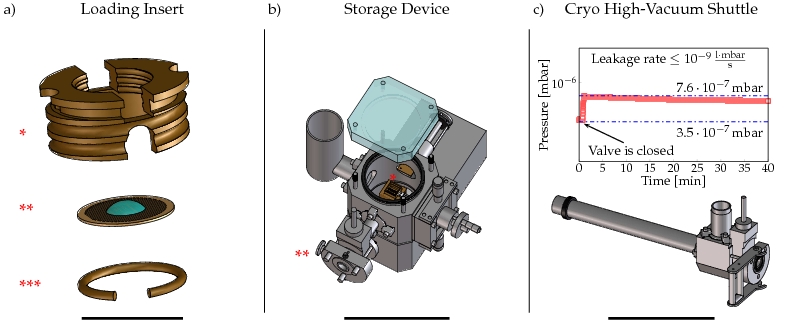
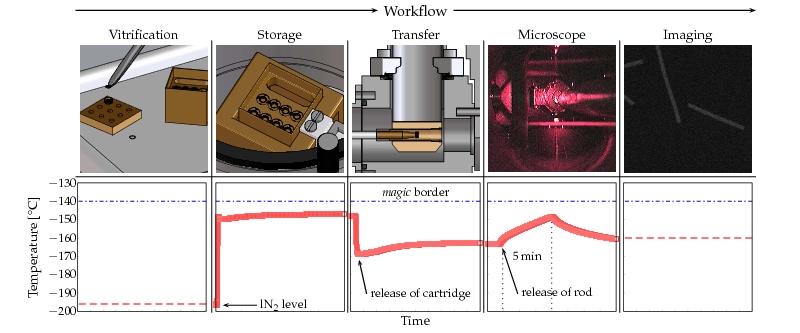
Here, we introduce an improved cryo high-vacuum transfer system (CHVTS) that allows for the first time to combine all kinds of cryogenic experiments. Moreover, we provide a solution that offers the highest degree of freedom in terms of connectivity of experiments (Fig.1). As shown in the detailed scheme of the CHVTS, our system is composed of cartridge, storage unit and cryo high-vacuum shuttle (Fig. 2). Once vitrified and mounted to cryo-holder cartridges (CT3500, Gatan) up to eight samples can be transferred to the storage unit. Thereafter, the cartridges can be transferred to the electron microscope or any other system extended by our docking device. A constant vacuum level of 7 ± 2 x 10-7 mbar and a temperature well below 133°K guarantee a contamination free transfer (see Fig. 3). Taken together, the CHVTS introduced in this work streamlines the handling of the frozen-hydrated specimen while solving for all problems generally associated with cryogenic investigations.
[1] J. Dubochet et al, Q. Rev. Biophys. 21 (1988), p. 129.
[2] L. Fitting Kourkoutis, J. M. Plitzko, and W. Baumeister, Annu. Rev. Mater. Res. 42 (2012), p. 33.
[3] S. G. Wolf, L. Houben and M. Elbaum, Nat. Methods online publication (2014), p. 1.
[4] C. Hagen et al, J. Struct. Biol. 177 (2012), p. 193.
[5] A. Rigort et al, J. Struct. Biol. 172 (2010), p. 169.
[6] S. Rubino et al, J. Struct. Biol. 180 (2012), p. 572
[7] E. Villa et al, Curr. Opin. Struc. Biol. 5 (2013), p. 771.
This research was supported by the DFG Grants RE 782/11-1,-2. Vladislav Krzyzanek acknowledges the support by the grant 14-20012S (GACR). We kindly acknowledge the help of the precision mechanical workshop, especially Martin Wensing. Additionally, we would like to thank Ulrike Keller for providing the EM grids, Harald Nüsse and Roger Wepf for numerous discussions.