IT-11-P-2745 Electron Holography and Electron Energy Loss Spectroscopy for Studying Electrochemical Reactions at Electrode/Electrolyte Interfaces in a Lithium-Ion Battery
It is essential that detailed knowledge about the electrochemical reactions near the interfaces between electrodes and electrolyte be obtained to find clues for the development of more efficient batteries [1]. For this purpose, measurement of electric potential distributions and direct observation of ion distributions are of fundamental importance. Here we report our results of observations of such phenomena in all-solid-state lithium-ion batteries.
Electron holography is a powerful technique to map electric potential distributions in working batteries [2,3], while electron energy loss spectroscopy (EELS) is an effective technique to directly detect lithium distributions. The two methods provide a powerful means of revealing the electrochemical reactions that occur at electrode/electrolyte interfaces.
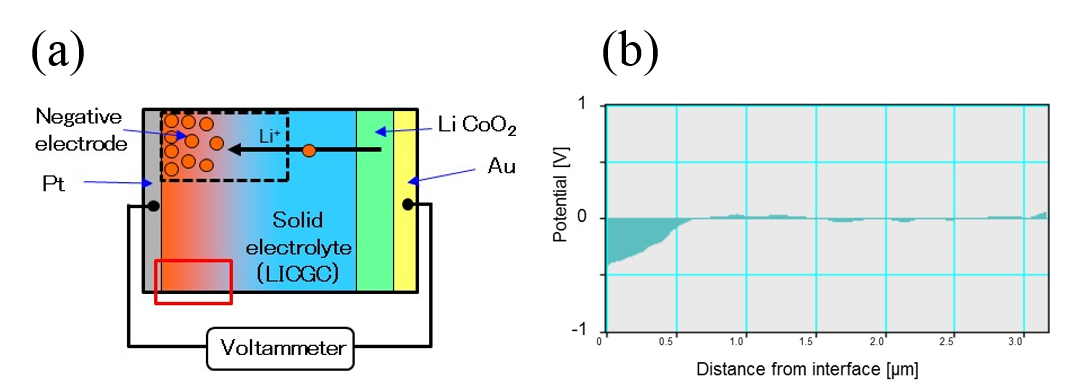
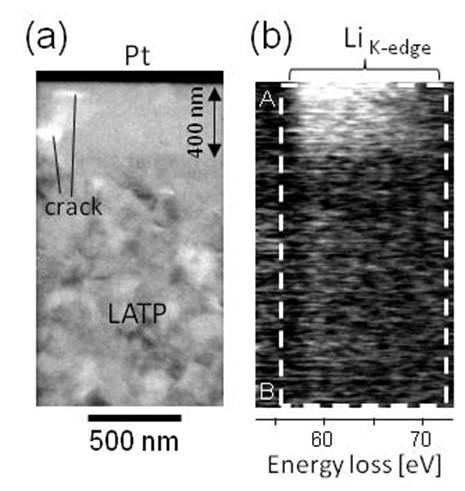
Figure 1(a) shows a schematic of the model battery used in our experiments. Fig. 1(b) shows the electric potential distribution measured at the negative side by electron holography after the first charge-discharge cycle. The negative potential indicates that a negative electrode region was formed in situ during this cycle [2]. Fig. 2(a) and 2(b) respectively show a transmission electron micrograph of a region near the negative electrode and corresponding spectrum obtained from Spatially Resolved Electron Energy Loss Spectroscopy (SR-EELS) measurements for a specimen that had undergone 50 charge-discharge cycles. This spectrum indicates that the in situ negative electrode contains excess lithium.
In summary, we have successfully observed distributions of electric potentials and lithium ions at the negative electrode side of an all-solid-state lithium-ion battery. These microscopy techniques provide new insights into the electrochemical reactions that take place in all-solid-state batteries during cycling, and should aid the design of high-performance devices.
References
[1] M. Armand and J.-M. Tarascon, Nature 451, 652-657 (2008).
[2] K. Yamamoto, et al., Angew. Chem. Int. Ed. 49, 4414-4417 (2010).
[3] K. Yamamoto, et al., Electrochem. Commun. 20, 113-116 (2012).
The authors would like to thank Dr. Y. Sugita and Mr. K. Miyahara of Chubu Electric Power Co., Inc., and Dr C. Fisher of Japan Fine Ceramics Center for valuable discussions. SR-EELS measurements were performed as part of the RISING project of NEDO, Japan. We are grateful to Profs. T. Abe, Y. Uchimoto and Z. Ogumi of Kyoto University for their encouragement and useful suggestions.