IT-7-O-2696 The Effect of Electron Beam on Aqueous Solution Composition during Liquid Cell Microscopy
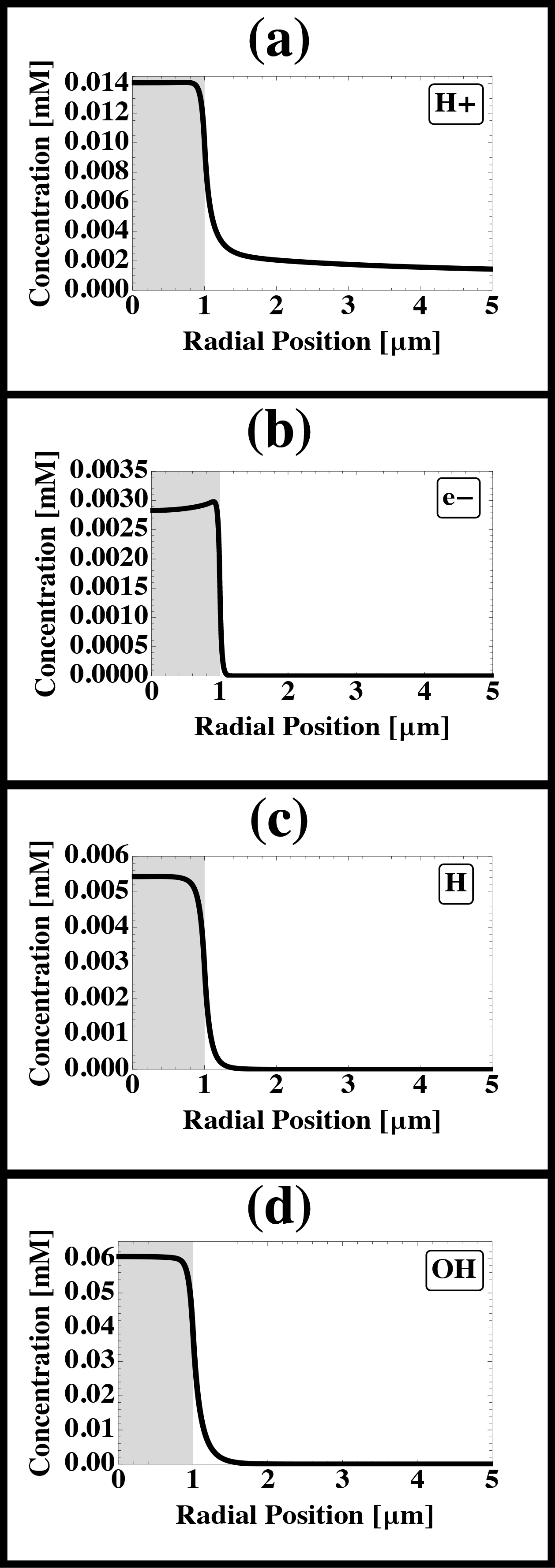
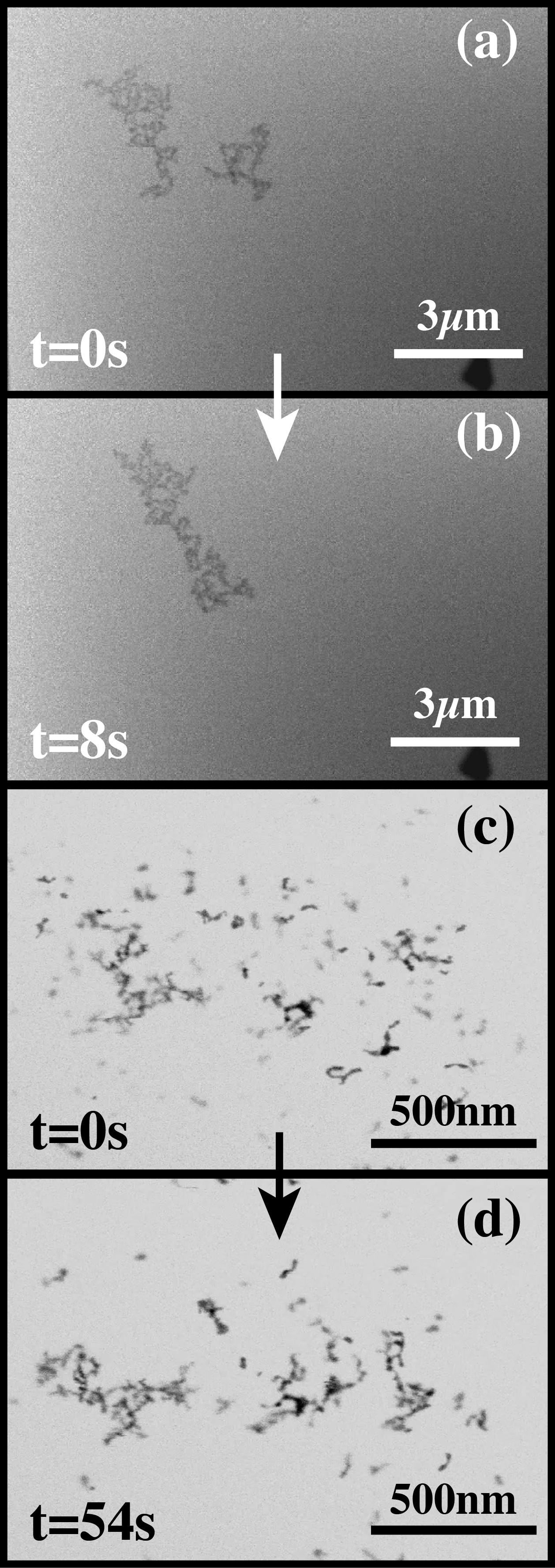
Liquid cell electron microscopy has emerged as a powerful tool for the real time imaging of objects suspended in liquids, and for characterizing processes that take place in liquids with the nanometer resolution of the electron microscope. However, as with all microscopy experiments, the electron beam interacts with the sample. Energy transferred from the fast-moving electrons to the irradiated medium causes excitation and ionization, resulting in the generation of radical and molecular species, which for water include eh (hydrated electrons), OH, H+, H2, O2, and H2O2. The hydrated electrons, oxidizing agents, and gaseous species can cause, respectively, reduction and precipitation of cations from solution, dissolution of metals, and nucleation and growth of bubbles. A quantitative understanding of electron beam-induced effects is critical to assessing whether the electron beam significantly affects the imaged phenomenon, so that we can correctly interpret experiments carried out with liquid cells; design experiments so as to minimize and mitigate unwanted effects; and take advantage of beam effects. We have developed a mathematical model to estimate radiolysis products during electron microscope imaging. The model includes the production of species by the electron beam, their destruction by reverse reactions, and their continued diffusion and reaction outside the irradiated region. We compute the concentrations of radiolysis products as functions of beam intensity, beam geometry, time, position, and solution initial composition (Fig. 1). We will describe this model and use its predictions to delineate various phenomena observed during liquid cell electron microscopy. For example, we predict that radiation chemistry causes large changes in pH within the irradiated region (Fig. 1a), and localized concentrations of reducing agents (Fig. 1b, c) and oxidizing agents. The pH of neat water can drop from 7 to 3.5 or lower within the imaged region under normal imaging conditions. Changes in pH can have significant effects on the phenomena under observation and may be the cause of aggregation of colloids (Fig. 2) that was observed during liquid cell imaging. We will compare the model with experiments carried out in a liquid cell, the nanoaquarium, at 300 kV in a Hitachi H9000 TEM and at 30 kV in an FEI Quanta FEG ESEM with a transmission detector, in each case imaging at 30 fps. The experiments and simulations suggest that liquid cell microscopy can provide a unique tool for studying radiolysis and for examining the behavior of materials subjected to high doses of radiation. We hope that the modeling tools described here will be useful for interpreting microscopy data obtained with liquid cells and for designing experiments that minimize unwanted effects.
The authors acknowledge funding, in part, from the National Science Foundation, grants 1129722 and 1066573.