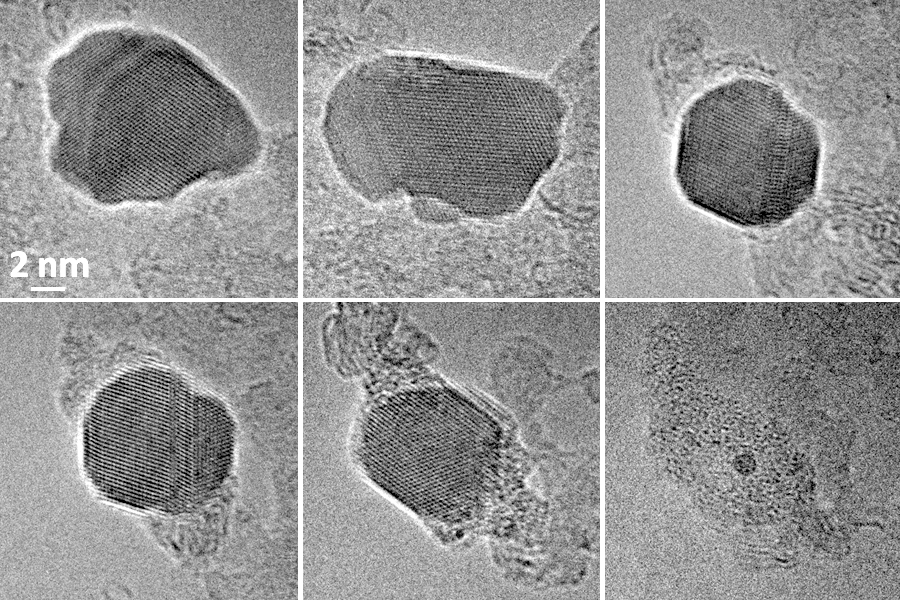
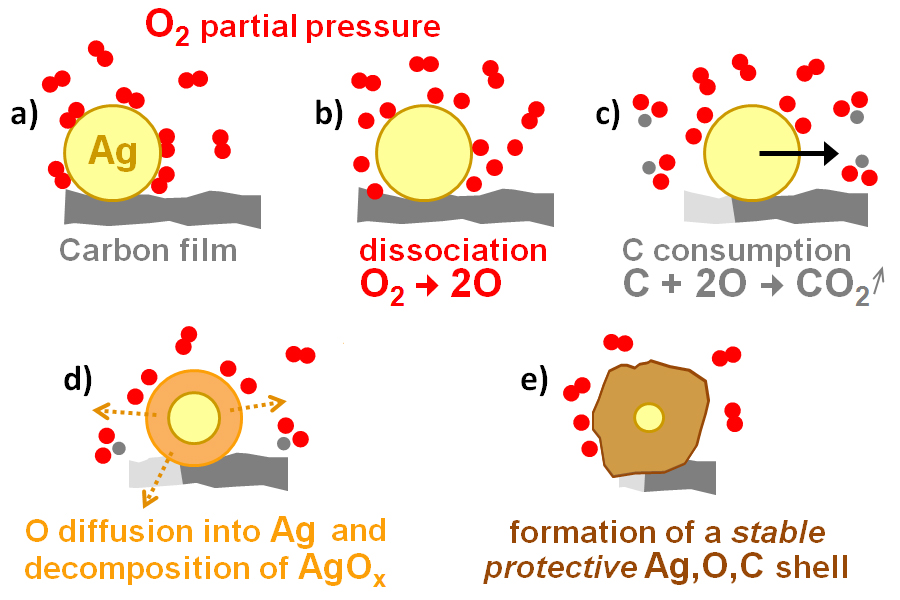
IT-6-P-2499 Carbon gasification by silver nanoparticles followed in situ at atomic resolution under oxygen partial pressure in an Environmental TEM (ETEM)
Generally, the gasification of hydrocarbon-based materials leads to the formation of syngas. Gasification can also be performed, at high temperature in oxidative environments, in presence of metal catalysts supported on solid carbon materials. Indeed, oxygen adsorbs and dissociates on the metal surface then interacts with the carbon at the interface with the metal leading to the formation of carbon dioxide; concurrently carbon is consumed and the metallic nanoparticle advances to maintain the interface with the carbon forming in this way a trench on the surface of the carbon. On structured materials such as graphite or graphene these trenches tend to be rather 2D [1,2] at the surface of the material. In this study we chose to study the gasification of a non-structured material (amorphous carbon) by silver-based nanoparticles in a Cs-corrected TITAN ETEM, 80-300 kV, recently installed at CLYM in Lyon. Samples were prepared according to a synthesis described in [4]; the silver based nanoparticles (NPs) hang on onto the supporting carbon films and gasification of this film is observed between 400 and 500°C under variable oxygen partial pressures (between 10-1 and 5 mbar). We could follow in real-time the dynamics of carbon gasification and catalyst evolution by high resolution imaging (Fig.1) unlike previous studies. In situ EELS yields complementary information (regarding oxygen) necessary to support the proposed mechanism deduced from our in situ study and schematically summarized in Fig.2: (i) at the beginning of the gasification experiment the NP have an hexagonal structure consistent with Ag2O or hexagonal-Ag (known to exist under the form of NPs or nanorods [5]) containing diluted oxygen (Fig.2 a-c); (ii) at a given moment during gasification the NP transforms to fcc-Ag, gasification slows down, the particle begins to shrink (which is consistent with the decomposition of a surface oxide) while a coating forms around it (Fig.2d); (iii) once the particle is completely coated gasification stops and the NP shrinking stops (Fig.2e).
[1] S.K. Shaikhutdinov, F.J. Cadete Santos Aires, Langmuir, 14 (1998) 3501.
[2] T.J. Booth et al., Nano Letters, 11 (2011) 2689.
[3] N. Severin et al., NanoLetters, 9(1) (2009) 457.
[4] S. Li et al., Chem. Comm. 49 (2013) 8507.
[5] I. Chakraborty et al., J. Phys.: Condens. Matter, 23 (2011) 325401.
Thanks are due to the CLYM (Centre Lyonnais de Microscopie, www.clym.fr) for its guidance in the ETEM project which was financially supported by the CNRS, the Région Rhône-Alpes, the ‘GrandLyon’ and the French Ministry of Research and Higher Education. The authors acknowledge S. Li, A. Tuel and D. Farrusseng for providing samples and L. Burel for her assistance in preparing them.