IT-5-P-2151 Non-stoichiometry and order-disorder in the SbxV1-xO2 (0<x<0.5) solid solution
~SbVO4 plays a key role in the catalyst for the ammoxidation of propane to acrylonitrile. Besides, it exhibits an amazing structural flexibility involving cation vacancies, changes in oxidation states and different degrees of order-disorder, ranging from Short Range Order (SRO) to periodic superstructures and structural modulations [1,2]. In this work we have studied the solid solution that ranges from VO2 to SbVO4 according to the following reaction stoichiometry:
[Sb2O3 + V2O5] + VO2 ---> SbxV1-xO2
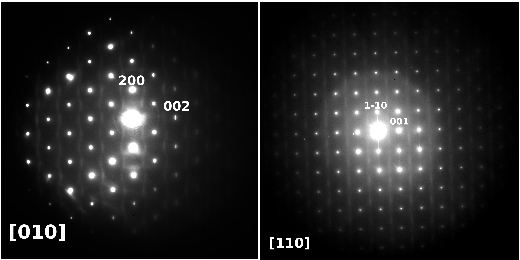
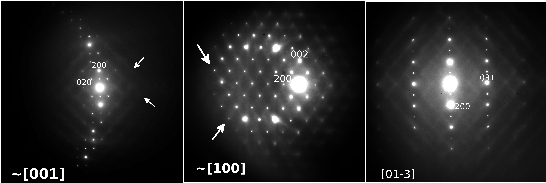
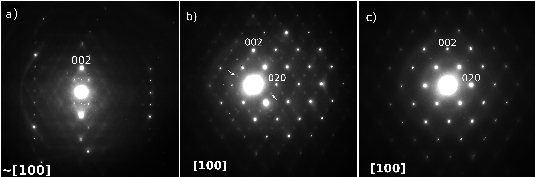
During the reaction all Sb3+ cations are oxidized to Sb5+ while all V5+ cations are reduced to V3+. This implies the following substitution in the basic rutile-type VO2 matrix: Sb5+ + V3+ <---> 2V4+. In this way we have been able to synthesize a whole solid solution SbxV1-xO2 ranging from SbVO4 (x=0.5) to Sb0.1V0.9O2 (x=0.1) by heating the stoichiometric amounts of Sb2O3, V2O5 and VO2 at 800ºC under argon atmosphere. In the Sb-richest phase, SbVO4, most of the vanadium is V3+, as confirmed by EELS spectroscopy, magnetic susceptibility and neutron diffraction, showing the latter magnetic ordering at TN < 50K. Electron diffraction shows the presence of intense SRO in the form of wavy two-dimensional sheets of diffuse intensity in the reciprocal space, see Fig. 1. HRTEM demonstrates that the SRO is due to low correlation between ...Sb-V-Sb-V... chains running along c. This SRO disappears at Sb0.33V0.67O2 and magnetic ordering happens at lower temperatures (TN~6K). For Sb0.25V0.75O2 compositions, a different type of SRO appears and its intensity increases as the amount of Sb decreases, Fig. 2. At Sb0.1V0.9O2 this SRO can also be observed by electron diffraction as very intense two-dimensional sheets of diffuse intensity forming a three-dimensional net of edge-sharing octahedra in reciprocal space. This phase presents a structural transition similar to that of VO2 but at lower temperature (51ºC). A phase transition can be observed by electron diffraction (Fig. 3) when heating with the electron beam from Short Range Order (SRO, diffuse lines) (a) to a two-fold superlattice (b) and back to SRO (c) as the temperature is lowered. During the whole series we range from SbVO4 with V3+ to VO2 with V<4+ and that big change in stoichiometry has been accommodated in a "soft way" through SRO mechanisms.
[1] A.R. Landa-Cánovas, J. Nilsson, S. Hansen, K. Staahl and A. Andersson. J. Solid State Chem.116, 369-377 (1995); [2] A. R. Landa-Cánovas, F. J. García-García, S. Hansen. Catalysis Today 158, (2010) 156.
Authors thank Spanish Government (project FLEXOCAT-MAT2011-27192) for financial support.