IT-6-P-1959 The Atmospheric Scanning Electron Microscope (ASEM) Observes Axonal Segmentation and Platelet Generation in Solution.
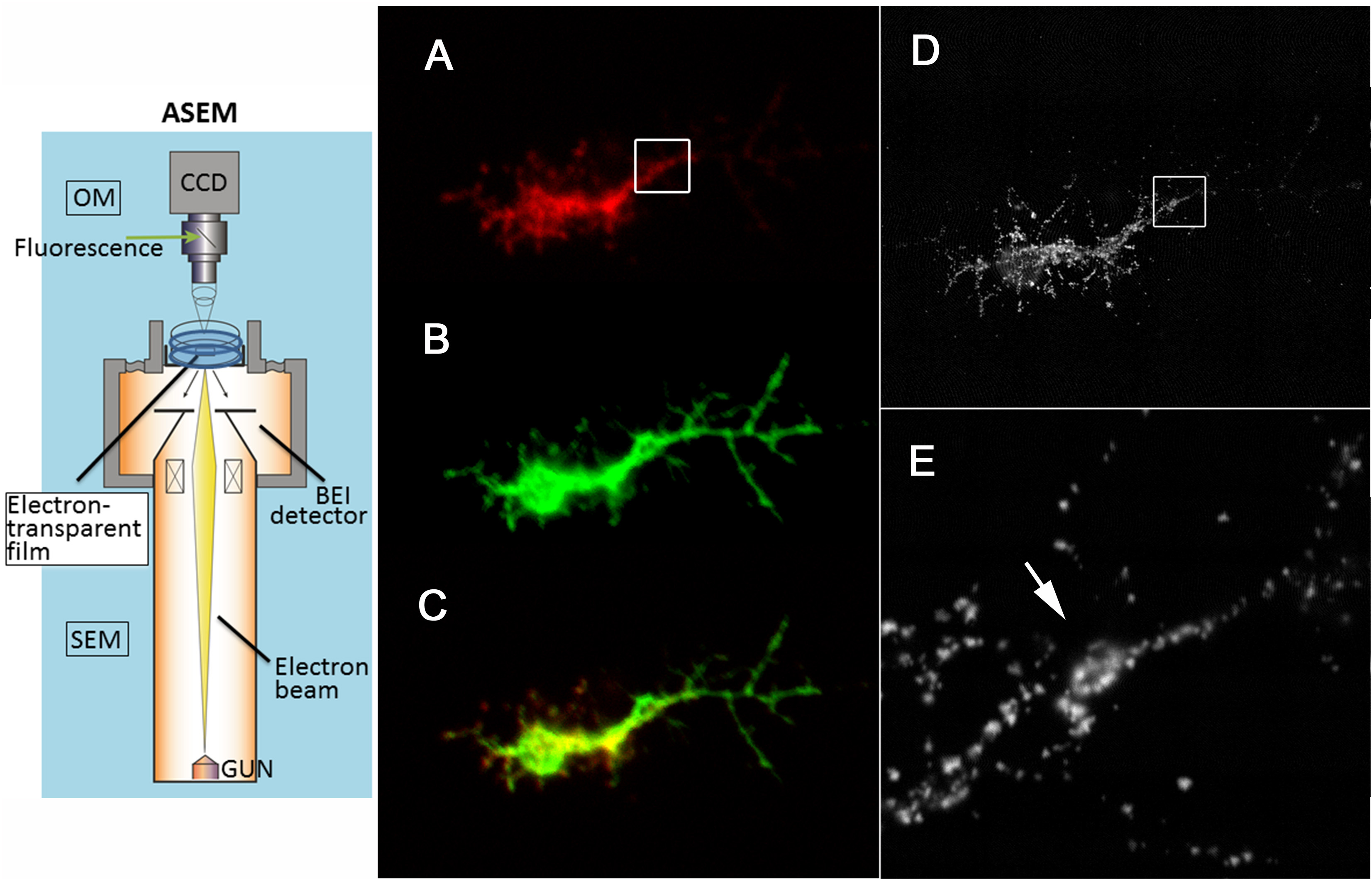
The new Atmospheric Scanning Electron Microscope (ASEM) is a Correlative Light-Electron Microscope (CLEM) [1]. Cell cultures of a few milliliters can be grown and differentiated directly in the removable ASEM dish, in a CO2 atmosphere if required. After fixation, the cells are imaged in situ, immersed in liquid and at atmospheric pressure, by optical microscopy (OM) and SEM in a fully correlative manner. Various dish coatings have been developed to increase the range of culturable cell types [2] allowing axonal segmentation and platelet generation to be investigated.
Axonal partitioning of neurons was correlated with specific cytoskeletal structures including microtubules. For this, isolated Drosophila primary neurons were grown on a poly-DL-ornithine-coated ASEM dish and immunolabeled for neuron markers HRP and BP102. Fluorescence microscopy demonstrated the localization of HRP in the whole axial fiber (Figure 1, B) and the specific localization of BP102 in the proximal region (A). ASEM revealed a hexagonal frame-like structure of BP102 at the boundaries of the most intra-axonal segments (E, arrow) [4], which has not been observed by OM. Two tubulin bundles running alongside one another make contact at the intra-axonal boundary and possibly elsewhere. Eight of the ten axons examined showed such contacts. In the other two axons, the immunolabeling was disconnected at the intra-axonal boundary [3]. This may mean that the two tubulin bundles are separated, although the possibility that labeling is prevented by proteins bound to the α-tubulins in this region cannot be excluded.
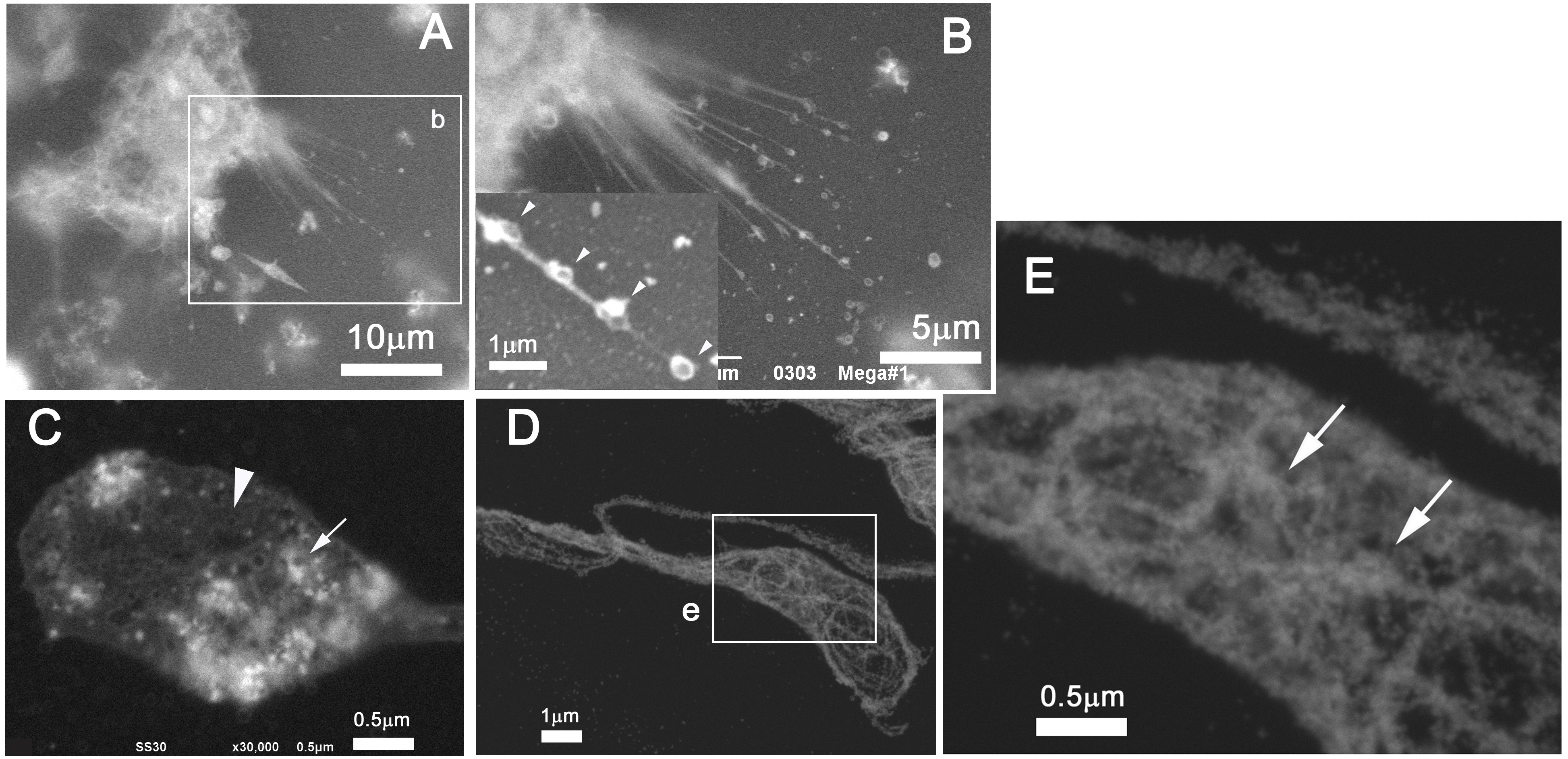
Mature megakaryocytes (MKs) generate beaded cell projections named proplatelets, and further shed off platelets, which are indispensable cellular components of blood for hemostasis. We cultured MKs on ASEM dish and fixed the cells at an appropriate timing captured with OM. The cells were stained with heavy metal solution and observed at high resolution with the inverted SEM. The pseudopodia extended beaded strings (Figure 2A-B), including vesicles (C). These vesicles are necessary for blood clot formation, which is related to cerebral or myocardial infarction under pathological conditions [4]. Immunolabeling of P-selectin indicates that the vesicles could be a-granules. Additional labeling of α-tubulin indicates their transportation on microtubules (D-E).
References
[1] H. Nishiyama et al, J Struct Biol 169 (2010), p. 438-449.
[2] Y. Maruyama et al, J Struct Biol 180 (2012), p. 259-270.
[3] Microsc. Microanal. in press, doi:10.1017/S1431927614000178
[4] Ultramicroscopy in press, (http://dx.doi.org/10.1016/j.ultramic.2013.10.010)
We thank Dr. Toshihiko Ogura at AIST for valuable discussions.