IT-6-P-1943 The Study of Ice Impurities Using the Environmental Scanning Electron Microscopy at Higher Pressures and Temperatures.
Natural ice and snow accumulate and concentrate significant amounts of impurities that can be stored or chemically transformed, and eventually released to the environment. The location of impurities and their interactions with the water molecules of ice have not yet been sufficiently clarified. The aim of this work is to observe an uranyl-salt brine layer on the ice surface using a back scattered electron detection and the ice surface morphology using a secondary electron detection under equilibrium conditions in a specimen chamber of environmental scanning electron microscope (ESEM).
Our specially modified ESEM AQUASEM II equipped with the YAG:Ce3+ backscattered electron detector, an ionization detector of secondary electrons, a special hydration system and a Peltier cooled stage was used. The pressures between 400-700 Pa, 50% water-vapor saturation, and the temperatures above 250 K were utilized in our experiments. At these conditions, the phenomena of etching and subsequent stripping of impurities are largely suppressed.
Our samples were frozen under atmospheric pressure on a silicon plate cooled by the Peltier cooled stage. The initial sample holder temperature was above –1°C. A droplet of pure water or the uranyl nitrate solution was exposed to freezing. The uranyl nitrate solution (0.01 M) acidified by perchloric acid to pH = 1 were used in our second experiment because the hydrolysis of UO22+ is suppressed and only a single species (i.e., a hydrated uranyl ion) is present under these conditions.
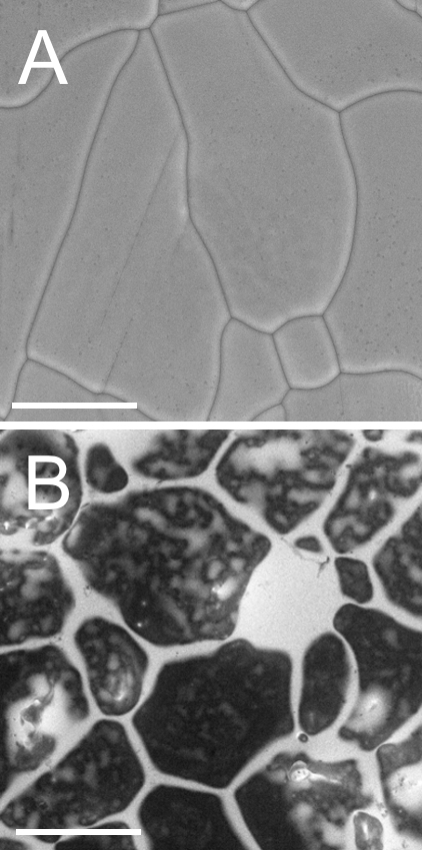
Figure 1A shows an ESEM image of the ice sample prepared by freezing of pure water under atmospheric pressure inside the specimen chamber. Different shapes and sizes (30–200 µm) of the ice grains can be distinguished. Due to the detection of secondary electrons (SE), which are sensitive mostly to the surface topography, the ice grain boundaries are visible as black lines with a bright halo. At this temperature, the ice crystals are covered with a disordered interface (also called quasi liquid layer), however it is too thin to be identified by ESEM. Since the amount of backscattered electrons (BSE) is related to the atomic number of the present elements, 92U-rich regions appear brighter, whereas the regions consisting of water molecules remain dark, see Figure 1B. The difference between pure ice and the frozen uranyl solution is largely manifested in the channels and pools of concentrated UO22+ solutions (bright) along with the individual ice grains (black). Pools are usually the largest at the triple junctions, although some may also be present on the ice surface. A liquid layer containing UO22+ was expected to be considerably more concentrated than the parent solution due to the freezing concentration effect.
This work was supported by the Grant Agency of the Czech Republic: grant No. GA 14-22777S.