IT-4-P-1917 New Preparation Method using Ionic Liquid for Fast and Reliable SEM Observation of Biological Specimens
For SEM observation, it is necessary for biological specimens to be treated with several types of preparation media to preserve their shape under vacuum. Ionic liquids are unique materials because of their natural incombustibility, non-volatility, and high ionic conductivity. Here, we used an ionic liquid to prepare samples for EM observation [1] [2]. The ionic liquid, IL1000 has been designed for use in EM sample preparation with a high level of safety and high solubility [3].
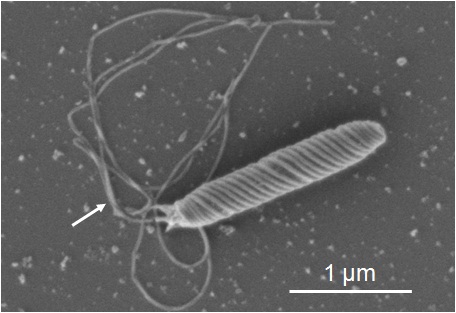
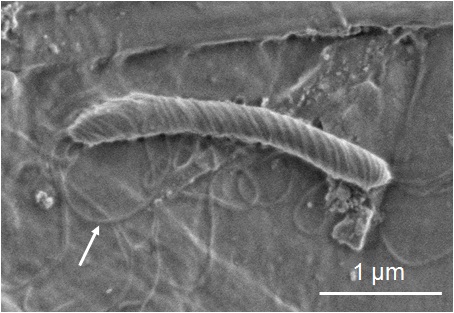
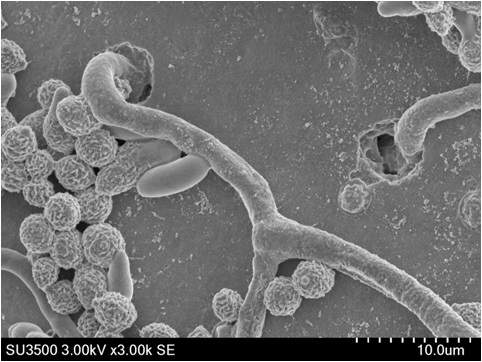
Figure 1 shows the SEM images of a Helicobactor Bilis sample prepared by conventional procedures. To preserve the flagella structure, the sample was immobilized on the cover slip coated with poly-L-lysine, and freeze-dried after fixation with 2 % glutaraldehyde (GA) in 0.1 M phosphate buffer and dehydrated with acetone in descending concentrations. The conventional sample preparation method takes approximately 8 hours. The surface of the cell body and some flagella are clearly observed (fig.1). On the other hand, in the ionic liquid (IL) method, the sample fixed by the buffered 2 % GA is immersed in 10 % IL1000 solution for 15 minutes and dropped onto filter paper. The sample is then directly transferred into the SEM without further drying. The resulting SEM image of this sample clearly shows the helical shape of the bacteria and flagella (fig. 2). Figure 3 shows the SEM images of mold growing on a rice cake. The square cut sample x5mm2, was immersed in the 10 % IL1000 solution for approximately 4 hours, and then directly transferred into the SEM. Fine threads protruding from the rice cake are clearly observed (fig.3) and the higher magnification image shows the clear and smooth surface of the spores.
These results indicate that the IL method for biological sample preparation greatly reduces preparation time, and is additionally better at preserving the sample’s original shape in the SEM.
References
[1] S. Kuwabata. et al, Chem. Lett., 35, p600-601. (2006)
[2] E.Nakazawa.et al, Proceeding of the Fifty-sixth Symposium of the Japanese Society of Microscope., 47-2, p92-95. (2013)
[3] K.Nimura. et al, Hitachi Hyoron., Vol.95, 9, p26-31. (2013)