IT-4-P-1912 Ionic Liquid Preparation for SEM Observation of Minute Crustacean
Electron microscopic observation is often possible along the surface of an arthropod, however applying common fixative media present difficulties when penetrating beyond the exoskeleton. Ionic liquids (ILs) are unique in that they are incombustible, non-volatile, and have high ionic conductivity. The application of ILs as part of sample preparation for EM were conducted with some particles dispersed in ILs(1) and observed by TEM, including IL wetted seaweed with observation by SEM(2). Recently, we have developed a new ionic liquid (IL) HILEM, IL1000 for EM observation. The ionic liquid, IL1000 has been designed for EM preparation, having both a high level of safety and high solubility, resulting in a high suitability for biological tissue preparation. In this experiment, minute crustaceans were immersed in 10 % IL1000 diluted with distilled water for a period of 60 minutes to 3 hours. For surface observation retention, any extra IL covering samples were removed by an absorbent cloth. The samples were observed by the Hitachi SU3500 at an acceleration voltage of 5 kV in high vacuum condition.
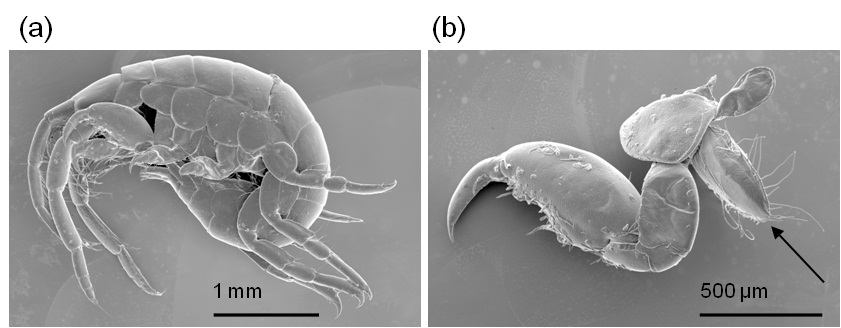
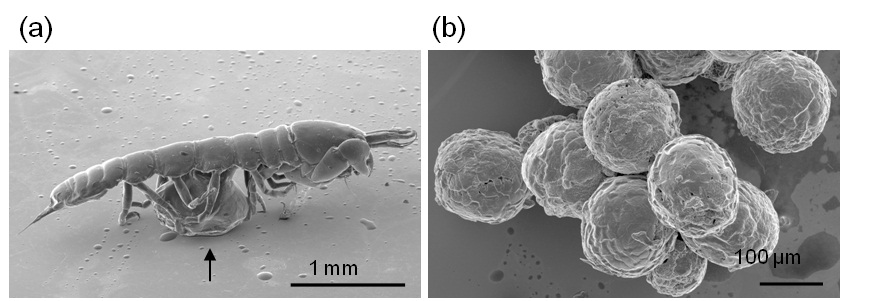
Figure 1 shows the secondary electron images of the minute crustaceans Gammaridea. Figure 1(a) is an image of the entire body of the Gammaridea, whose individual appendages are not easily distinguishable due to the overlapping of appendages. The imaged sample was removed from the SEM and tweezers were used with the aid of a binocular to separate its appendages from the body. Figure 1(b) is the separated appendage (the second thoracic appendage) of the Gammaridea and the attached organ shown by arrow in Figure 1(b). The organ shown functioned in protecting the egg, therefore it was determined that this specimen is female. The results show that the IL can deeply penetrate the specimen which aids electron conductivity inside the specimen. Figure 2 is the secondary electron images of the Tanaidacea. Figure 2(a) is the whole image of the Tanaidacea orientated to observe the ventral side. The female of the Tanaidacea has the brood chamber in the thorax (arrow) region. As aforementioned above, the observed specimen was removed from the SEM to separate its brood chamber. Figure 2(b) is a SEM image of the eggs that were removed from the brood chamber. Since the sample was soaked by IL, the sample is resistant to rapid dehydration while under vacuum, including soft materialsuch as eggs can be preserved by this technique. We emphasize that the IL1000 is a useful media for SEM observation of some soft and delicate biological material.
References
[1] E. Nakazawa. et al, Proceedings of the sixty-fourth Annual Meeting of The Japan Society of Microscopy. p 136 (2008)
[2] S. Kuwabata. et al, Kenbikyo, 44: p 61-63. (2009)