IT-3-O-1814 Large parallelization of STED nanoscopy using optical lattices
Recent developments in super-resolution microscopy techniques achieved nanometer scale resolution and showed great potential in live cell imaging. STED (Stimulated Emission Depletion) and more generally RESOLFT (REversible Saturable OpticaL Fluorescence Transitions) need parallelization in order to fully benefit from this spatial resolution for fast wide-field imaging. An approach for parallelization is based on structured illumination pattern. RESOLF parallelization has been proposed using 1D interference pattern, but the resolution improvement is only obtained along one direction. Recently methods for massive parallelization of RESOLFT with photo-switchable proteins and STED nanoscopy based on the use of 2D structured illumination have been reported. Larger field of view could be achieved for parallelized RESOLFT using photo-switchable fluorescent proteins, because it requires less intensity to switch. However, protein switching is a relatively slow on-off process (~10 ms), which sets a limit to the imaging acquisition rate. Moreover RESOLFT with photo-switchable fluorescent proteins is constrained in its versatility by the need for genetic modification and transfection.
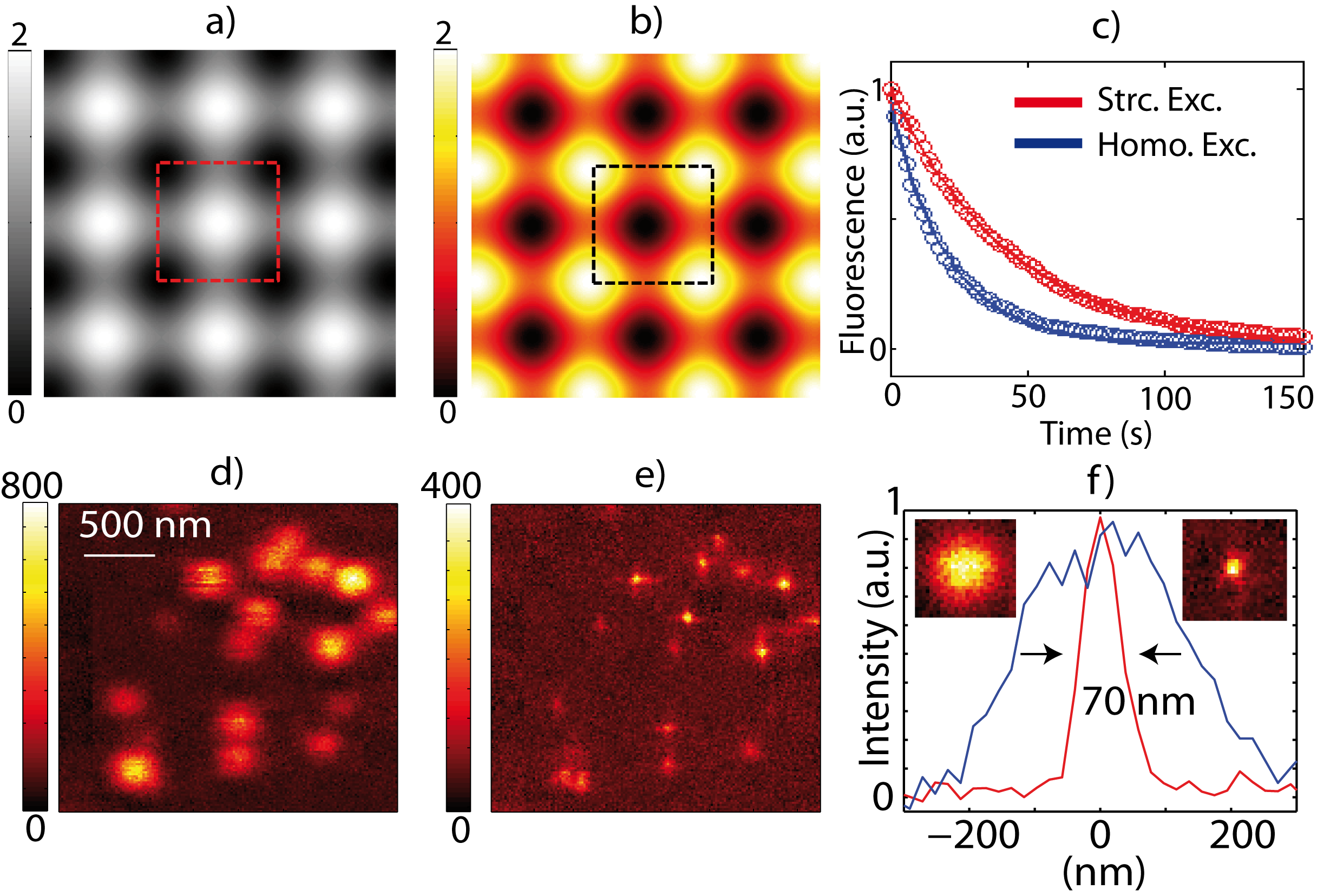
We show how well-designed optical lattices, created by multi-beam interference can provide efficient depletion patterns with moderate laser power and can be used for large parallelization of STED, so far the most important and widely used RESOLFT technique. The stimulated emission depletion being an ultrafast on-off switching process (~1 ns), its imaging speed is therefore only limited by the number of detected photons and fast large field of view super-resolution imaging can be achieved. With optical lattices, acquisition of large field of view super-resolved images only requires scanning over a single unit cell of the optical lattice which can be as small as 290 nm x 290 nm. STED images of 2.9 µm x 2.9 µm with resolution down to 70 nm are obtained at a frame rate of 12.5 images/s (figure 1).
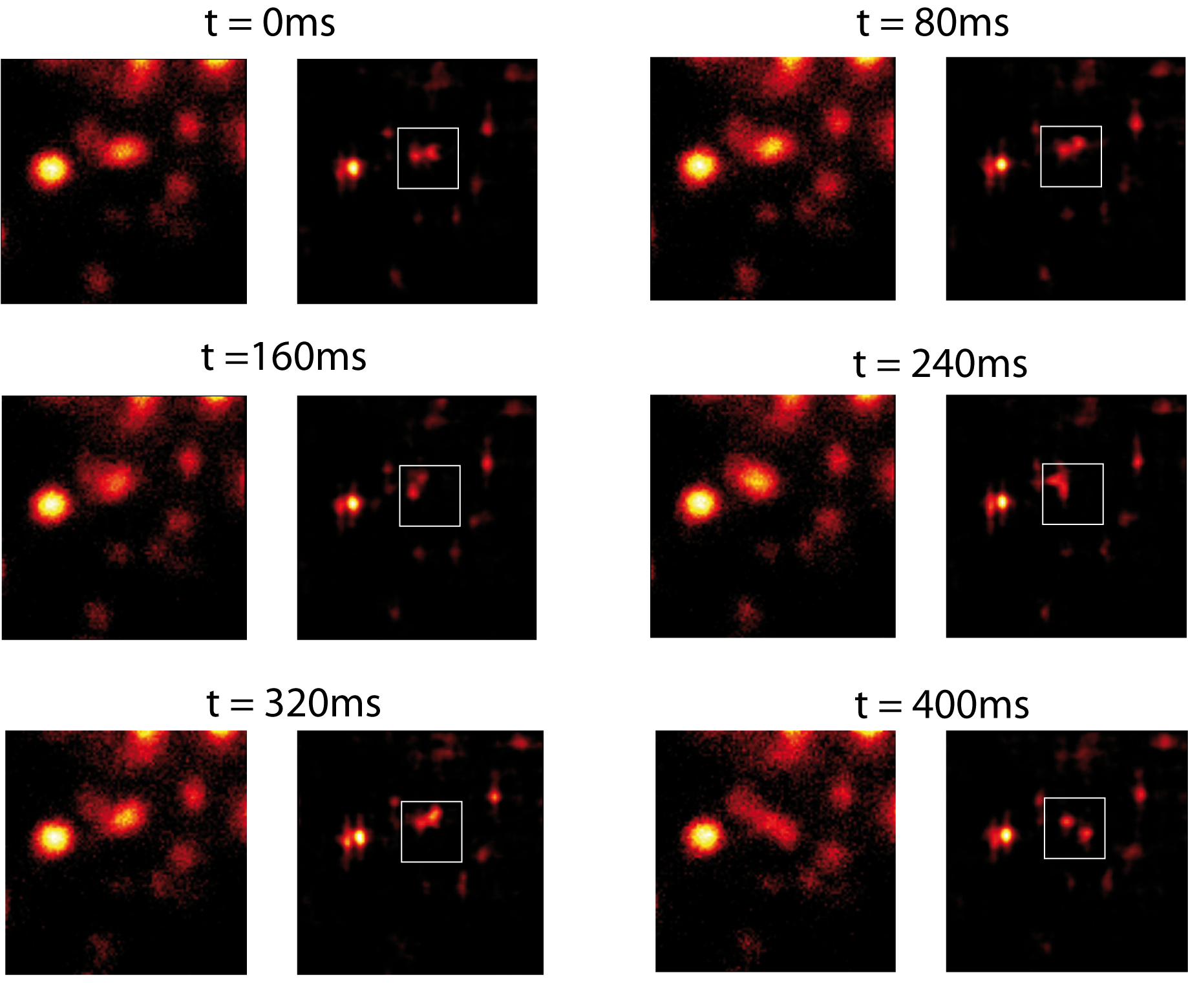
Photobleaching might be a constraint in STED nanoscopy when recording a large number of frames. We reduce the photobleaching (i.e. the probability of a molecule to get promoted to a highly excited and reactive levels), by structuring both excitation and depletion beams. In this case, we found a decay time two times longer than in the homogeneous excitation case. We demonstraste the high-speed capability of our STED microscope by imaging the movement of 20 nm fluorescence particles in a Carbopol gel [1] (figure 2). We clearly show that recording of fast relative movement of two particles separated by a distance well below the diffraction limit.
Reference :
[1] B. Yang et al.,“Large parallelization of STED nanoscopy using optical lattices”, Optics Express, In Press (2014).
We acknowledge financial support from the ANR, Région Aquitaine, the French Ministry of Education and Research, the ERC and FranceBioImaging (Grant N° ANR-10-INSB-04-01).