IT-6-P-1598 Quasi in situ TEM characterization of Ni reduction in regenerated Ni/alumina catalysts
Ni/alumina are active and selective catalysts for the selective hydrogenation of pyrolyse gasoline produced by steam cracker. This reaction allows removing alkadiene and alkenyl aromatics from C5+ fraction, without hydrogenating the aromatic rings and forming saturated hydrocarbons. In order to extend catalyst life, regeneration of spent catalysts followed by a reduction step can be applied to obtain a metallic and redispersed catalyst with activity as close as possible to the one of the fresh catalyst.
In this work, such reduction of a regenerated Ni/alumina catalyst containing reoxidized Ni particles was studied. The morphological evolution of the nanoparticles during reduction was followed by “quasi in situ” TEM, using a special Gatan HHST4004 “Heating Environmental Cell Holder” equipped with an ex-situ reactor and allowing the observation of the same zone before and after thermal treatment, in controlled atmospheric conditions [1].
After regeneration, all Ni in the sample was in oxidic form, as shown by FFT and SAED analyses. Two kinds of Ni oxide particles were observed, namely (i) small well-dispersed particles with sizes centered around 11 nm and (ii) large hollow particles (up to 30 nm in diameter), probably formed by Kirkendall effect from the initial metallic particles, as previously reported for Co Fischer-Tropsch catalysts [2]. After 2 hours of reduction at 410°C in Ar-5% H2 (treatment performed in the sample holder reactor), a modification of the morphology of the large hollow particles was observed. Thus, the hollow spheres broke during the reduction into a group of smaller Ni° particles forming a ring-like aggregate whose size is the same as for the initial hollow particle (figures 1 and 2). Besides, small nanoparticles with sizes nearly as above were still present but smaller nanometric particles also appeared. Moreover, all nickel was fully reduced, whatever the type of the nanoparticle.
These observations are in good accordance with previous results obtained for Ni catalysts used in the partial oxidation of methane [3] and for cobalt FT catalyst [2]. In summary, the present study shows that regeneration and reduction of a spent Ni/alumina catalyst used in selective hydrogenation of pyrolyse gasoline leads to a reduced well-dispersed catalyst. Besides, this study highlights the interest of the "quasi in situ" TEM technique to follow morphological evolutions during activation and/or regeneration treatments of supported catalysts.
[1] E. Sayah, D. Brouri, P. Massiani Catalysis Today 218– 219 (2013) 10–17
[2] C. J. Weststrate and al., Top Catal (2011) 54: 811-816
[3] S. Chenna and al., ChemCatChem 3 (2011) 1051-1059
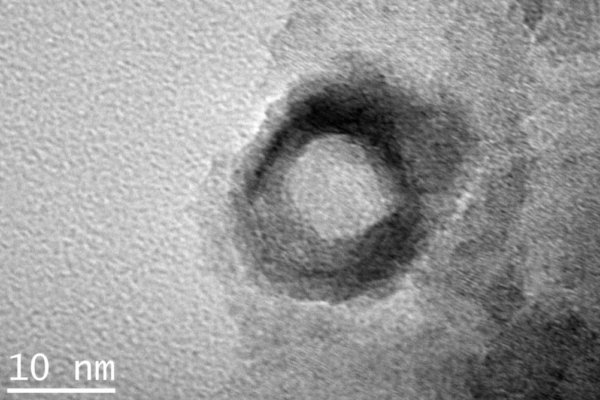
Fig. 1: Hollow NiO particle in the regenerated catalyst |

Fig. 2: Same zone - Ring-like aggregate of Ni° particles after 2h of reduction at 410°C under Ar/5%H2 |